1. AQP1
AQP1 is found in dermal fibroblasts and vascular endothelial cells and has also been detected in melanocytes, located in the SB of the epidermis [
37]. The main function of AQP1 is played in the vascular endothelial cells, where it mediates the exchange of water between the blood and dermis to maintain hydration. Less well elucidated is the physiological meaning in fibroblasts and melanocytes. In fibroblasts, AQP1 has been found to be upregulated during periods of hypertonic stress [
38]. A similar increase in AQP1 expression has been postulated in melanocytes during conditions of osmotic stress; however, further work is needed [
39]. AQP1-mediated water transport has also been suggested to occur in keratinocyte migration. Cell migration was restored in keratinocytes derived from AQP3-deficient mice by infecting the AQP1-depleted cells with either AQP3 or AQP1 [
40]. The influx of water through either AQP1 or AQP3 was speculated to provide the hydraulic pressure needed to extend processes for cell movement [
40].
2. AQP3
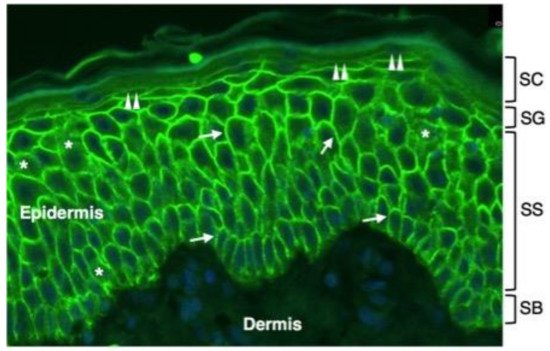
AQP3 is expressed in keratinocytes from the SB to the SG of the epidermis where it is localized at the plasma membrane (Figure 1).
Figure 1. Immunofluorescence distribution of AQP3 in normal human epidermis. Shown is immunofluorescence analysis of AQP3 labelling in the epidermis of a normal human subject. Skin tissue was formalin-fixed and paraffin-embedded. Sections were stained using an anti-human AQP3 antibody recognizing AQP3 (Thermo Fisher Scientific, Monza, Italy). Cell nuclei were stained with DAPI (blue fluorescence). Strong AQP3 immunoreactivity (green fluorescence) is seen over the plasma membrane of stratum basale (SB) and stratum spinosum (SS) keratinocytes (arrows). Weak intracellular immunoreactivity is observed in the intracellular compartment (asterisks). The plasma membrane immunostaining of SG keratinocytes is lower than the one of the underlying epidermal layers (double arrowheads). No immunofluorescence is seen in the stratum corneum (SC) of the epidermis and in the dermis.
At a much lower extent than the plasma membrane level, AQP3 is also detected in the intracellular compartment of keratinocytes (
Figure 2). This pattern is consistent with an in vitro study reporting the strong downregulation of AQP3 mRNA levels as basal keratinocytes reach late differentiation [
41]. Plasma membrane AQP3 has been reported to be associated with phospholipase D2 (PLD2) in caveolin-rich membrane microdomains, leading to the hypothesis that glycerol imported through AQP3 is used by PLD2 in the transphosphatidylation reaction that generates phosphatidylglycerol (PG), a phospholipid that acts as a signalling molecule to mediate early epidermal keratinocyte differentiation. Manipulation of this signalling module was reported to block keratinocyte proliferation and increase differentiation [
42]. The mechanisms through which the AQP3 expression is regulated in the epidermis are just starting to be understood. A recent study with keratinocytes and mouse skin ex vivo reported that histone deacetylase-3 (HDAC3) has a role in modulating AQP3 expression. The levels of AQP3 mRNA and protein were found as increased after the selective inhibition of HDAC3, suggesting that in epidermal keratinocytes under basal conditions, HDAC3 suppresses AQP3 expression [
43]. A role for p53 transcription factors in increasing AQP3 has been also suggested [
43]. However, neither the possibility that the enhanced AQP3 expression in response to HDAC inhibition results from a generalized increase in gene transcription by means of effects on chromatin structure, through the stimulation of the activity of one or more transcription factors including p53 or another family member, nor a combination of these processes can be ruled out. The AQP3 promoter contains a p53 response element that can also be bound and activated by p73 [
44]. The activation of PPARγ has been shown to lead to a marked increase of the AQP3 mRNA in both undifferentiated and differentiated cultured human keratinocytes (CHKs). The increase in AQP3 transcript by PPARγ agonists occurred in a dose- and time-dependent manner. The increase in AQP3 mRNA level was followed by an augmentation in AQP3 protein in undifferentiated keratinocytes and a considerable increase in glycerol uptake [
45]. The topical application of ciglitazone, a PPARγ activator, also increased AQP3 expression in mouse skin in vivo. Similar effects on AQP3 expression were seen after activating other nuclear hormone receptors such as liver X receptors (LXR), retinoic acid receptors (RAR), and retinoid X receptors (RXR) [
45]. Consistent with this observation, Yang and colleagues reported that PPARs contribute to the HDAC inhibitor-induced increase in AQP3 levels as PPAR antagonists were seen to prevent the HDAC inhibitor-dependent elevation of AQP3 levels. PPARγ upregulation was accompanied by the HDAC inhibition-stimulated AQP3 mRNA and protein expression [
46]. However, further work is needed to evaluate whether the inhibition of HDAC leads to an increase in the accessibility of the gene promoter of AQP3 or modifies the acetylation of PPAR to stimulate its transcriptional activity. AQP3 possesses a N-linked glycosylation site at loop C, and in several cell types, this consensus seems to be functional, as besides the core protein of about 28 kDa, glycosylated forms with molecular masses ranging between 40 and 60 kDa are also present. AQP3 appears to be glycosylated also in keratinocytes [
47] where, as for other AQP homologues, proper glycosylation may affect the function and subcellular localization of the protein [
48]. In murine epidermal keratinocytes, AQP3 was downregulated by differentiating agents such as 1,25-dihydroxyvitamin D3 and high levels of extracellular calcium (1 mM) [
41]. This was accompanied by a reduction of keratinocyte glycerol uptake. However, glycerol uptake is increased by moderate extracellular calcium (125 µM) [
49] at a level known to promote keratinocyte differentiation in vitro [
50]. Qin and coworkers reported in vitro evidence that 125 µM extracellular calcium decreases the non-glycosylated form of AQP3 and increases the glycosylated form of the protein [
42]. If glycosylation favours the plasma membrane localization as seen for AQP2 in kidney cells [
51], this fits with the reported pattern of localization of AQP3 in the suprabasal skin layers of human [
52] and mouse [
42] skin. This possibility does not seem to fit with a study by another group using cultured human keratinocytes where no effect of elevated Ca
2+ concentration on AQP3 protein levels was observed [
45]. However, in the same work, it was shown that the effect of PPAR agonists in increasing AQP3 was limited to the glycosylated form of the protein, suggesting that, also in human keratinocytes, differentiating agents increase glycosylated AQP3 levels. Phosphorylation may be another post-translational modification regulating the subcellular localization of AQP3 as occurs for AQP2 in the principal cells of renal-collecting ducts’ translocation to the plasma membrane. However, no evidence exists supporting this eventuality. In keratinocytes, AQP3 has been reported to translocate to the plasma membrane upon exposure to osmotic stress [
53]. A final post-translational modification that may affect it is protein acetylation. Renal AQP3 was shown to be acetylated on lysine 282 [
54], and a similar modification may occur in keratinocyte AQP3, influencing its subcellular localization and/or function. Hence, the promotion of lysine acetylation by HDAC inhibition might exert actions on AQP3 in addition to its effects on expression. Mechanisms other than post-translational modifications may also regulate AQP3. A basolateral targeting sequence (YLLR) is found in AQP3 from multiple species [
55]. Although the current literature provides interesting information about the regulation of AQP3 in the skin, additional studies are needed to fully assess the posttranslational modifications of AQP3 and the role of these modifications on the subcellular localization and function of this channel.
Key roles have been ascribed to AQP3 in various processes underlying keratinocyte function. The importance of AQP3 in skin is also indicated by the dysfunctions observed in a number of human skin diseases. A role for AQP3 is often suggested in keratinocyte differentiation. By an in vitro study, Bollag and colleagues suggested a function of AQP3 in early mouse keratinocytes differentiation [
56]. AQP3-mediated import of glycerol inhibited cultured keratinocytes proliferation, leading to the appealing hypothesis that AQP3 is pivotal in arresting the growth of basal keratinocytes after they move into the first differentiated layer of the SS. This possibility was in line with a subsequent study with non-melanoma skin cancer cells characterised by excessive proliferation. AQP3 was greatly decreased in the lesions compared to the overlying normal-appearing epidermis [
57]. A role for AQP3 in promoting keratinocyte differentiation was also proposed after co-expressing AQP3 with constructs in which the promoters of keratinocyte differentiation markers drove the expression of a reporter enzyme [
56] and in a study where re-expression of the protein in AQP3-knockout keratinocytes led to increase of the mRNA and protein levels of differentiation markers either alone and/or in combination with an elevated concentration of Ca
2+ [
47]. Knockdown of AQP3 inhibited the expression of keratin 10 in keratinocytes exposed to high Ca
2+ levels to induce differentiation [
58]. The same study also suggested a role for AQP3 in promoting keratinocyte viability. The association between keratinocytes’ AQP3 levels and early differentiation through Notch1 signalling was reported by Guo and colleagues [
10]. In spite of the above evidence on the role of AQP3 in keratinocytes, differentiation remains rather controversial. After knocking down AQP3, Verkman and coworkers found no changes in the levels of differentiation markers in human keratinocytes induced to differentiate by high Ca
2+ concentration [
59]. Moreover, no changes in the basal expression of keratinocyte differentiation markers were seen in the epidermis of knockout mice lacking AQP3. Based on their previous studies indicating a physical and functional association between AQP3 and PLD2, Bollag and colleagues proposed that the ability of AQP3 to induce keratinocyte differentiation depends on its interaction with this lipid-metabolising enzyme phospholipase [
60]. As mentioned above, these authors suggested that PLD2 converts the AQP3-transported glycerol to PG via transphosphatidylation, promoting keratinocyte differentiation through the AQP3/PLD2/PG signalling pathway [
56]. However, further work is needed to unravel the apparent discrepancies regarding the role of AQP3 as a glycerol channel in skin keratinocyte differentiation. It should also be considered that glycerol can cross the plasma membrane by moving by simple diffusion through the phospholipid bilayer [
61].
An important role for AQP3 has been suggested in keratinocyte proliferation by a series of studies conducted by Verkman and colleagues [
8,
40,
59,
62,
63]. Work with AQP3 knockout mice indicated that this role is not played under basal conditions in vivo since the mice with the ablation of AQP3 showed similar epidermal thicknesses and layer numbers as well as proliferation compared with wild-type mice [
62]. In a subsequent study, the
Aqp3−/− mice showed reduced tumour formation when submitted to topical application of a carcinogen followed by treatment with a tumour promoter [
63]. Moreover, after tumour promoter activation, the
Aqp3 knockout mice showed less epidermal thickening and a smaller increase in the number of proliferating cells compared with the wild-type mice. The reduced keratinocyte proliferation was interpreted as due to decreased cellular ATP levels caused by the lack of AQP3 and the consequent reduced cellular uptake and metabolism of glycerol. Indeed, reduced epidermal glycerol content was found in AQP3-deficient mice [
62], and supplementation with glycerol corrected the defect in keratinocyte proliferation induced by wounding [
40,
63]. In a study with normal human keratinocytes AQP3 upregulation was also seen to increase glycerol uptake, keratin 5 and 14 expression, and cell growth, whereas AQP3 silencing inhibited proliferation in response to the cytokine CCL17 [
8]. A similar result was seen in vivo in AQP3-ablated mice challenged with retinoic acid [
59]. Comparable results were obtained by Guo and coworkers who found that AQP3 knockdown diminished human keratinocyte proliferation and increased the expression of early (keratin 10), intermediate (involucrin), and late (filaggrin) differentiation markers [
10]. Roles for AQP3 in promoting cell proliferation have also been reported in several cancers (for review see [
64]). AQP3 has also been found to promote epithelial-mesenchymal transition in gastric cancer cells [
65] and to facilitate epidermal cell migration during wound healing. Knockdown of AQP3 in normal human keratinocytes decreased glycerol uptake, scratch wound healing, and fetal bovine serum-induced migration in vitro [
40]. Keratinocytes isolated from AQP3-deficient mice showed reduced migration compared with cells originating from wild-type mice [
40]. Adenoviral-mediated expression of either AQP1, an orthodox aquaporin water channel with no permeability to glycerol, or AQP3 was able to restore normal epidermal cell migration [
40]. This suggests that the effect of AQP3 in skin keratinocyte migration relates to the facilitation of water movement rather than the transport of glycerol. However, the facilitation of hydrogen peroxide uptake cannot be ruled out since AQP3 has good peroxiporin activity, and convincing evidence exists indicating relevance of AQP3-mediated H
2O
2 import in cell migration [
66,
67].
As anticipated above, AQP3 also exerts an important function in skin wound healing, a role in line with its involvement in cell proliferation and migration. AQP3-ablated mice showed delayed wound healing of full-thickness skin wounds accompanied by decreased keratinocyte proliferation, and this impairment was rescued through glycerol supplementation [
40]. Consistent with this result, AQP3 expression was decreased in the wounds of diabetic rats with impaired wound healing [
68]. However, whether AQP3 is also involved in human skin wound healing needs to be proved since rodents are not ideal models for human skin wound healing (rodent wounds heal mainly by contraction, whereas human wounds heal primarily by re-epithelialization). Moreover, wound healing is an extremely complex process that involves many other cell types, thus making it even more difficult to assess the exact role of AQP3 in its development. Besides keratinocytes, indeed, endothelial cells and myofibroblasts represent the major cellular actors in the wound healing process, especially regarding inflammation and wound contraction, respectively [
69]. Therefore, if on one hand endothelial cells take part to the initial cellular crosstalk that promotes local inflammation, vasodilation, and haemostasis, thus facilitating tissue repair and growth factors concentration, myofibroblasts are required at the end of the process in order to reconstitute an efficient extracellular matrix as well as to restore tissue deficiency when re-epithelialization is not possible or sufficient [
70,
71]. Nevertheless, due to the central role of re-epithelialization in human wound healing and the relevance of AQP3 in promoting the proliferation and migration of human keratinocytes, it is reasonable to hypothesize a consistent role of AQP3 in human skin wound healing as well. Convincing evidence exists indicating an important role of AQP3 in skin hydration. AQP3-deficient mice showed reduced SC hydration compared to the wild-type control mice [
62,
72], and the difference between control and
Aqp3−/− knockout mice was abolished after the exposure of the mice to low (10%) humidity [
72]. No differences were seen in SC morphology, thickness, lipid content, or levels of a variety of metabolites, whereas a decrease in SC and epidermal glycerol content was observed [
72]. The SC hydration defect was corrected by restoring the epidermal glycerol levels [
73]. The pivotal role of AQP3 in skin hydration is also indicated by its circadian rhythm of expression, and skin hydration correlates with these cyclical AQP3 levels [
74]. Glycerol generation from triglyceride in sebaceous glands is fundamental for SC hydration, as also indicated by a study with the asebia mouse model where defect in sebum production leads to a reduction in epidermal glycerol content and abnormal SC hydration, which in turn leads to hyperkeratosis (epidermal thickening), epidermal hyperplasia, and mast cell activation. This phenotype can be resolved by the topical administration of glycerol but not urea or water [
75], suggesting a key physiological role in skin function and the involvement of AQP3 in preserving epidermal glycerol content and SC water-holding capacity. In a mouse model of streptozotocin (STZ)-induced diabetes, changes in epidermal AQP3 expression have been invoked to explain the skin dryness (xeroderma) accompanying diabetes, and the disruption of the circadian rhythm was suggested as a possible mechanism through which STZ-induced diabetes contributes to AQP3 downregulation [
76].
AQP3 has also been reported to be important in the function of the epidermal water permeability barrier. Although basal skin barrier function was not impaired, AQP3-ablated mice showed an approximately two-fold delay in the repair of their water permeability barrier compared with the wild-type control as measured by transepidermal water loss after tape stripping [
62]. Again, as occurred for other altered skin functions, oral glycerol administration restored the impaired barrier recovery of AQP3-deficient mice and even accelerated recovery in the wild-type control [
62]. In line with these results, Bollag and colleagues found an accelerated water permeability barrier repair in a transgenic mouse where AQP3 was selectively overexpressed in the epidermis under the control of the keratin 1 promoter [
42].
AQP3 is also expressed in dermal-resident T cells, where it was suggested to regulate their trafficking in cutaneous immune reactions after observing that AQP3-ablated mice are defective in the development of hapten-induced contact hypersensitivity [
67]. The impaired trafficking of antigen-primed T cells to the hapten-challenged skin was attributed to the AQP3-mediated H
2O
2 uptake required for the chemokine-dependent migration of T cells.
3. AQP5
Eccrine sweat glands are composed of single tubular structures containing acinar cells and ductal cells. Mouse, rat, and human eccrine sweat gland secretory and excretory cells express AQP5 that, upon stimulation, traffics at their apical membrane [
36,
77,
78]. Acinar cells secrete a primary fluid rich in ions and water whose composition is modified by reabsorption by the ductal cells [
79]. The role of AQP5 in eccrine sweat glands remains an open debate due to variable data acquired with different AQP5 knockout mice strains and methods to assess the secretion [
36,
80]. Zhou and coworkers are the unique authors detecting AQP5 in keratinocytes where they suggested a role for this AQP in the balance between epidermal keratinocyte proliferation and differentiation [
81]. However, further studies are needed to evaluate if the expression of AQP5 in keratinocytes in vivo is physiologically relevant and, eventually, clarify its role in the epidermis.
The presence of AQP5 in human axillary sweat glands has been reported, and upregulation of this orthodox AQP was detected in axillary sweat glands of primary focal hyperhidrosis (PFH) patients. PFH is a sweat disorder characterised by excessive sweating in specific body areas, such as the palms, axillae, feet, or forehead, where the eccrine sweat glands are concentrated [
82]. This is consistent with a work by Ma and coworkers who used a mouse model and reported that topiramate reduced sweat secretion along with a diminished AQP5 expression by sweat glands, suggesting that AQP5 may be involved in topiramate-induced hypohidrosis [
83]. These observations conflicted with a previous study where no significant difference in AQP5 expression was observed in the palmoplantar skin of healthy subjects and patients with a form of palmoplantar hyperhidrosis [
84]. Thus, the main role of AQP5 in human sweat glands remains to be fully clarified; further investigation is needed to increase the understanding of the pathogenic mechanisms of hyperhydrosis and the hypothesized implication of AQP5.
4. AQP7
Dendritic cells (DCs) feature the ability to present antigen and exert a critical role in the induction of the acquired immune response. The immune system of the skin relies on a rich network of antigen-presenting DCs that localise in the epidermis and the dermis. Skin DCs are distinct into three subsets, epidermal Langerhans cells (LCs) and Langerin- or Langerin+ dermal DCs (dDCs). LCs and dDCs uptake antigen and subsequently migrate to regional draining lymph nodes, where they activate naive T cells by initiating the immune response. A study using AQP7-deficient mice and isolated LCs and dDCs from mouse skin found that AQP7 is functionally expressed in mouse LCs and dDCs and plays roles in antigen uptake, cell migration, and the subsequent initiation of an immune reaction [
35]. AQP7 was suggested to play an important role in allergy induction and immune surveillance in the skin and, likely, in other tissues in which DCs are localized.
5. AQP9
AQP9 is a homologue of AQP3 with broad selectivity and is predominantly expressed in leukocytes [
85] and the liver [
61]. AQP9 was detected in the upper SG of the human and mouse epidermis [
86]. AQP9 knockout mice did not show any apparent defect, including wound healing [
86]. AQP9 mRNA expression was only detected in cultured, differentiated normal human epidermal keratinocytes [
87]. Stimulation with retinoic acid, a potent stimulator of keratinocyte differentiation, increased AQP3 expression and downregulated AQP9 expression, suggesting that AQP9 expression in epidermal keratinocytes undergoes a different regulation compared to that of AQP3 [
88]. The treatment of cultured keratinocytes with the keratinocyte differentiating agent VD3 also significantly increased AQP9 expression, an observation that has led to the hypothesis that AQP9 plays a role in highly differentiated keratinocytes. In human epidermis, AQP9 and occludin, a tight junction marker, co-localize in the upper stratum granulosum [
88]. Considering that tight junctions function as a paracellular barrier against small molecules, besides having a role in the terminal differentiation of keratinocytes, another function for AQP9 in human epidermis may be that of constituting a transcellular route for the movement of solutes of biological relevance, such as glycerol and urea, into and out of the skin.
The involvement of neutrophil AQP9 in hapten-induced contact hypersensitivity has been shown in a murine model of skin allergic-contact dermatitis using
Aqp9−/− knockout mice [
89]. AQP9 was therefore suggested to be required for the development of sensitization during cutaneous acquired immune responses via regulating neutrophil function.
6. AQP10
AQP10 is another aquaglyceroporin detected in the epidermal keratinocytes. By in vivo studies, AQP10 has been localized to the SC of the human epidermis [
90]. AQP10 is believed to share similar meaning as AQP3, as it may play a role in the barrier function of the skin due to its expression in the SC [
39,
90]. The involvement of AQP10 in the pompholyx has also been suggested [
91], although further studies are needed to precisely address this possibility.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23074020