Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The outbreak of coronavirus disease (COVID-19), which comes with several comorbidities, was declared a pandemic in early 2020 by World Health Organization (WHO). Glucocorticoids that are used in severe cases of COVID-19 as therapeutic measures may lead to opportunistic fungal infections in such patients. Mucormycosis is one of these infections and mostly occurs in immune-compromised patients such as those who undergo transplant surgeries.
- corticosteroids
- antifungal therapy
- COVID-19
1. Clinical Manifestations
Currently, COVID-19 patients encounter more mucormycosis cases as compared to patients undergoing any other diseases inclusive of clinical manifestations as 1. Rhinocerebral, 2. Pulmonary, 3. Central Nervous System (CNS), 4. Cutaneous, 5. Gastrointestinal, 6. Disseminated, and 7. Miscellaneous (e.g., bones, kidney, oral, heart, and mediastinum) (Figure 1).
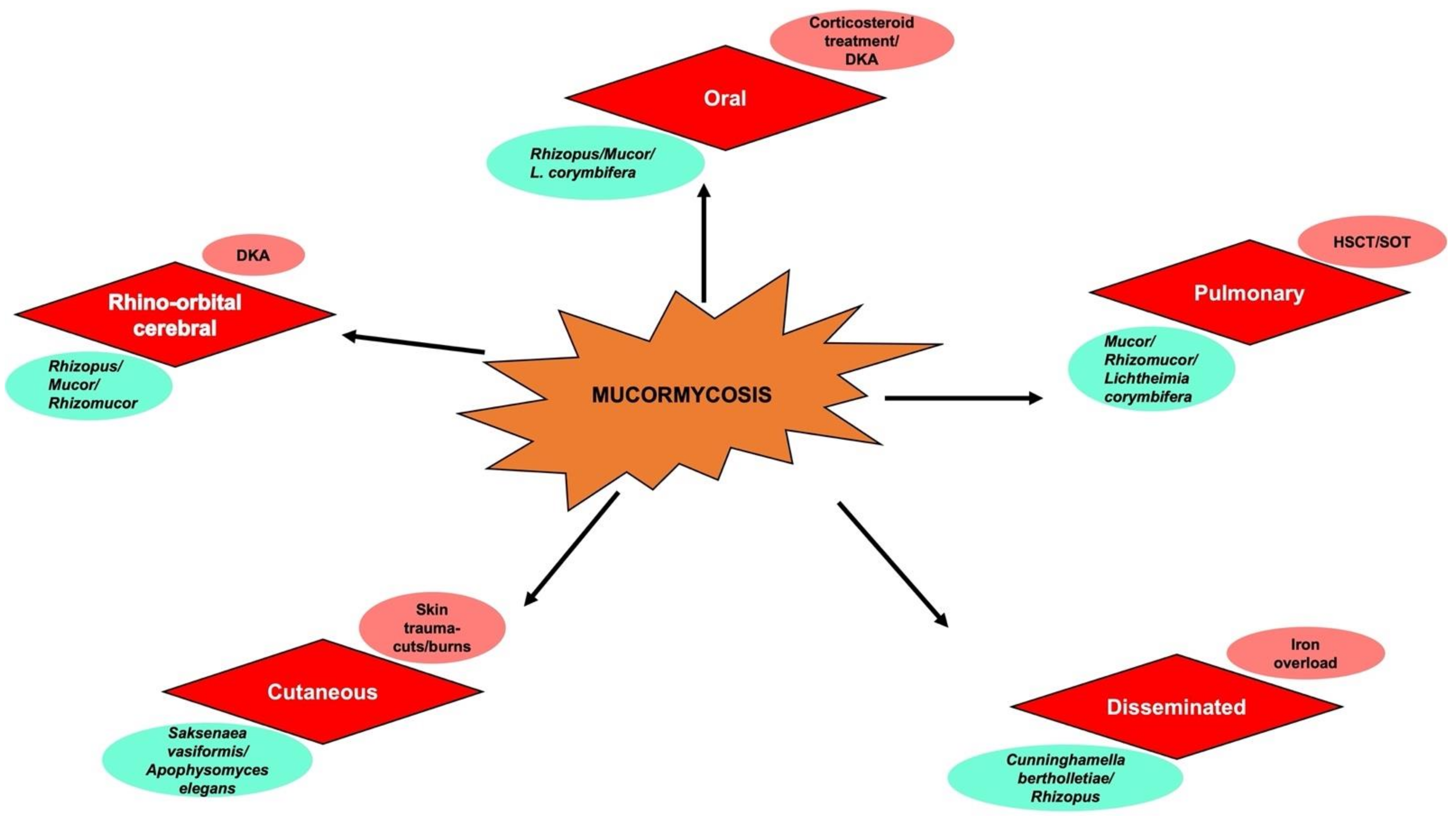
Figure 1. Schematic representation of different types of mucormycosis (in red), underlying risk factors (in pink), and corresponding causative species (in cyan green). DKA—Diabetic Ketoacidosis; HSCT—Hematopoeitic stem cell transplant; SOT—Solid organ transplant.
1.1. Rhinocereberal Mucormycosis
Facial pain, headache, and fever are some of the common symptoms in cases of rhinocerebral mucormycosis (ROCM). ROCM in COVID-19 patients has been presented as a frequent and severe condition with the majority of the cases being identified in India (approximately 70%) and having diabetes as an underlying condition. In a recent study of 80 CAM patients, the association of rhino-orbital cerebral infection with extension into the CNS was confirmed in 37% of the patients. In addition, mortality in patients with ROCM with confirmed CNS involvement was found to be more than two-fold greater than in those without CNS involvement [1]. The related symptoms of CNS involvement are described in the next subsection.
In cases where the infection reaches the nasal turbinates, orbital cellulitis, extraocular muscle paresis, proptosis, and chemosis have been reported. The results of standard roentgenograms and computed tomography (CT) scans display prominent soft-tissue swelling as signs of infection possibly due to the necrosis of frontal lobes extending to posterior brain regions. However, bone destruction is only evident at the later stages of infection marking progressive mucormycosis [2]. Since Rhizopus spp. utilizes deferoxamine as a siderophore to supply previously unavailable iron for the fungus growth [3], the growth of R. oryzae in DKA patients due to iron release from binding proteins may lead to the development of rhinocerebral mucormycosis.
1.2. CNS Involvement
The most common route of infection of the CNS is known to be via the nose or paranasal sinuses. In intravenous (i.v.) drug abusers, it has been reported to spread hematogenously. Related predispositions such as brain infarcts were also observed in neutropenic patients and drug abusers infected with the human immunodeficiency virus; however, the likely cause of the underlying condition for the development of mucormycosis is neither reported to be the virus nor the i.v. inoculation itself [4][5]. Likely, conditions such as neutropenia, bone marrow transplantation, and viral infections are interrelated to eventually cause mucormycosis. At times, inflammatory soft tissue infiltration extends to subcutaneous facial tissue along intratemporal and temporal fossae which may lead to the involvement of the CNS [6]. This evidence largely testifies the severity of the mucormycosis infection and possible inter-related effects associated with the emergence of COVID-19 disease.
1.3. Other Forms of Mucormycosis
Leukemic patients and those undergoing bone marrow transplants are most likely to develop pulmonary mucormycosis due to neutropenia. The common symptoms include fever, dyspnea, and chest pain. The inflammatory cells are usually absent and only abnormalities such as tissue necrosis and hemorrhage are evident on roentgenograms due to which the actual intensity of the infection and related damage is higher than the observation [2]. Considering the phenotypical similarities observed in different infectious routes, the careful examination of the upper and lower respiratory tracts of the concerned patients is advisable.
Another common form of mycoses, cutaneous mucormycosis, is caused by fungi deposited into the skin and sometimes to the deeper subcutaneous layer via the use of contaminated Elastoplast bandages. The spores from the contaminated material applied to the skin are able to proliferate and invade both cutaneous and subcutaneous sites [2].
Gastrointestinal mucormycosis has been yet another common issue in countries with the problem of severe malnutrition. The involvement of the stomach, ileum, and colon has been reported more commonly than that of the rest of the digestive system [4]. Patients normally present with the nonspecific signs and symptoms of an intra-abdominal abscess followed by the possibility of hematochezia [5]. Gastrointestinal mucormycosis is most often diagnosed after the patient has died of the infection, but has also been presented with acute gastrointestinal symptoms after recovery from COVID-19 disease [6].
1.4. Oral Manifestations: A Dentist’s Perspective
In dentistry, invasive mucormycosis gains increasing interest because of its initial manifestation in the facial and oral tissue often leading to a black necrotic eschar on the palate where ischemic necrosis of the mucoperiosteum with bony denudation may take place [7]. Besides this, ulcers have also been reported on the gingiva, lips, alveolar ridge, cheeks, tongue, and mandible parts [8][9][10].
Palatal necrosis develops from fungal spores entering through the nose or mouth to invade the orbit or open wounds [11][12] and spreading to the paranasal sinuses, skin of the face, cribriform plate, and brain through vascular channels [13][14]. The penetration of the arterial walls through fungal entry in the blood causes collateral endothelial damage resulting in intravascular thrombosis, infarction, and tissue necrosis [13][14][15][16]. In diabetic patients, this vascular clogging may cause severe local tissue ischemia and increased susceptibility to infection [16][17].
CAM affects the sinuses in the majority of cases (>80%), followed by the rhino-orbital cerebral region in at least 50% of the reported cases. The role of dentists is critical since they may be the first clinicians to be presented with oral manifestations in high-risk patients, including those around the rhinomaxillary or rhinocerebral areas. The symptoms may range from mucosal discoloration, swelling, and bone exposure to atypical symptoms such as sinus pain, facial pain, and unanticipated odontalgia of otherwise sound teeth [18].
In this scenario, surgical intervention is both an aid and an alternative to the poor penetration of antifungal agents at the site of infection due to blood vessel thrombosis and resulting tissue necrosis. Hence, debridement of necrotic tissues is a critical step performed by dental surgeons. In many cases, patients who did not undergo surgical debridement of the lesion have been observed to show a relatively higher mortality rate [19]. According to a recent retrospective review of patients with ROCM, the use of intraoperative frozen sections to delineate the margins of infected tissues through calcofluor fluorescence microscopy is suggested so the uninvolved tissues can be spared from the debridement [20][21]. A nasal endoscopy may not always be an optimized measure to locate the lesion in the sinuses by restricting the access for debridement. Therefore, the Caldwell-Luc operation can be performed as an alternative surgical modality to successfully remove the necrotic tissue of the sinus. Often, an incision is made just above the root surface of the first premolar and molar, and the infected tissue is removed while splinting of teeth is performed, to immobilize them.
2. Treatment
The successful treatment of mucormycosis depends on a synchronized surgical and medical approach. Furthermore, surgical debridement is necessary to remove any of the devitalized tissue and requires immediate action within a few days after diagnosis. DKA, on the other hand, could be rapidly treated by cutting down on immunosuppressive drugs, whenever possible.
2.1. Primary Diagnosis and Prevention
The advantage of using early initiation of polyene antifungal therapy within five days of diagnosis is a significant improvement as compared to the initiation of the same at ≥6 days after diagnosis (83% vs. 49% survival). Hence, an early diagnosis to commence active antifungal therapy is highly recommended wherein a quantitative PCR system has emerged to be highly promising. Besides, MRIs and CT scans are useful for the specific detection of orbital and CNS involvement. Additionally, CT scans are also useful for the early detection of pulmonary mucormycosis, particularly in patients with cancer [18][19]. Other diagnostic advances that can prove to be successful in present times were mentioned in the previous section.
Removal of underlying defects in the host’s defense is a critical step when treating patients with mucormycosis. Firstly, where applicable, immunosuppressive medications, particularly corticosteroids, should be dose-reduced or stopped. The recommended daily dosages of corticosteroids such as dexamethasone, prednisolone, and methylprednisolone are 6 mg, 40 mg, and 32 mg, respectively, for about 10 days [22]. Secondly, aggressive management to restore normal blood sugar levels (euglycemia) and consequently, acid-base status in diabetic ketoacidosis conditions, is required [19]. Finally, wherever feasible, the administration of iron and blood transfusions should be avoided.
2.2. Antifungal Measures
Early diagnosis and aggressive medical management of underlying clinical manifestations may lead to a <20% death rate [19]. In this context, antifungal drugs have garnered a lot of attention; i.v. administration of conventional amphotericin B at dosages of 1 mg(/kg/d) is recommended, although a dosage of 1.5 mg(/kg/d) may be required for the treatment of patients who have aggressive and rapidly progressive infections [22]. Certainly, the patient’s response, underlying disease, and nature and degree of amphotericin B–related toxicity are some of the factors to be considered in deciding the success rate of any therapeutic measures. The addition of rifampin or tetracycline along with amphotericin B to enhance antifungal activity and administrative therapy with hyperbaric oxygen are some of the unproven measures [23][24] that are not recommended for general use in most patients. The susceptibility tests to decipher the kind of antifungal drug or combination of drugs, such as itraconazole or fluconazole, to administer in therapeutic regimens are also not warranted, due to the lack of standardized procedures. For instance, the combination of liposomal amphotericin B (LAmB, recommended as first-line therapy for nephrotoxic patients, 5mg/kg/d) and posaconazole has shown synergistic results against fungal hyphae formation [25] whereas the neutropenic patients or individuals with graft-versus-host disease could be administered with oral posaconazole as prophylactic doses for the management of the disease. Additionally, posaconazole is an economical alternative with an easy route of administration; however, the main drawback lies with the reduced absorption rate. Moreover, a combination of polyene-caspofungin therapy displayed a significant success in patients with rhino-orbital and ROCM as compared with polyene monotherapy [20]. Isavuconazole, on the other hand, offers some advantages with its high tolerance, lower side effects, bioavailability, and reduced drug-drug interaction, but is yet to be established in further studies [26]. It has been reported to be effective against Rhizopus delemar species [22].
The use of iron chelators, such as deferiprone and deferasirox, to treat iron-overloading transfusion-dependent anemias has been approved by the U.S. FDA [27][28] and has displayed improved survival in rodents with mucormycosis [29]. Deferasirox exhibited time-dependent killing as fungicidal effects occurred at 12–24 h of drug exposure with an MIC90 of 6.25 μg/mL [30]. The oral administration of deferasirox is rather simple and convenient but with the limited data availability, it must be administered cautiously at intervals (20 mg/kg/d for 2–4 weeks) with regular monitoring of renal and hepatic function. Again, the combination of deferasirox and LAmB therapy remarkably improved survival [31], with a 100-fold decrease in brain fungal burden compared with the monotherapy for which the former has been used off-label as adjunctive therapy for mucormycosis patients [23].
2.3. Salvage Therapy
As mentioned above, posaconazole or deferasirox are reasonable salvage options for patients refractory to polyene therapy and even appear to be a safer alternative for a longer period of administration up to a few months. In the light of increasing evidence for the use of G-CSF-mobilized granulocyte transfusions as additional support for persistently neutropenic patients, the administration of GM-CSF or IFN-γ in non-neutropenic patients has proven to aggravate host response against pro-fungal effects. In a recent murine study, not only did the addition of GM-CSF to LAmB therapy improved the survival of mucormycosis mouse models [32], but also the combined therapy of recombinant G-CSF and GM-CSF, or recombinant IFN-γ in conjunction with LAmB, proved successful for the treatment of mucormycosis [31][32].
2.4. Nanomedicine
Antifungal drugs display limitations like optimal dosage, infusion-related side effects, and high risk of nephrotoxicity, among other therapy-limiting effects. Recently, the use of nanosystems for delivery of Am-B via oral, topical, or even pulmonary routes appears to have become a promising alternative and is currently under development [32]. The lipid formulation of drugs such as AmB or nystatin can be augmented to reduce the toxicity of the conventional drugs owing to their hydrodynamic size and easy route of administration. The recent studies highlighted the use of silver nanoparticles (AgNPs), Zirconium oxide nanoparticles (ZrO2NPs), and nano-emulsion NB-201, which exhibit antifungal properties with higher toxicity against Mucorales due to the presence of corresponding moieties and relatively low toxicity in human cells [26].
This entry is adapted from the peer-reviewed paper 10.3390/biomed2020017
References
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.-P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The emergence of COVID-19 associated mucormycosis: A review of cases from 18 countries. Lancet Microbe 2022, in press.
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012, 54 (Suppl. 1), S23–S34.
- De Locht, M.; Boelaert, J.R.; Schneider, Y.J. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem. Pharmacol. 1994, 47, 1843–1850.
- Singh, R.P.; Gupta, N.; Kaur, T.; Gupta, A. Rare case of gastrointestinal mucormycosis with colonic perforation in an immunocompetent patient with COVID-19. BMJ Case Rep. 2021, 14, e244096.
- Chiang, T.-H.; Lee, Y.-W.; Tan, J.-H.; Kao, C.-C.; Chang, C.-C.; Fang, K.-C. Mucormycosis causing massive lower gastrointestinal bleeding: A case report. BMC Gastroenterol. 2021, 21, 272.
- Chikley, A.; Ben-Ami, R.; Kontoyiannis, D.P. Mucormycosis of the Central Nervous System. J. Fungi 2019, 5, 59.
- Ramon, Y.; Oberman, M.; Horowitz, I.; Freedman, A. Extensive maxillary sequestration resulting from rhinocerebral mucormycosis. J. Oral Surg. 1977, 35, 989–991.
- Jayachandran, S.; Krithika, C. Mucormycosis presenting as palatal perforation. Indian J. Dent. Res. 2006, 17, 139–142.
- Woo, S.B.; Setterfield, J.F.; Greenberg, M.S. Ulcerative, vesiculous and bullous lesion. In Burket's Oral Medicine, 11th ed.; Greenberg, M.S., Glick, M., Ship, J.A., Eds.; BC Decker Inc.: Hamilton, ON, Canada, 2008; pp. 74–75.
- Bonifaz, A.; Macias, B.; Paredes-Farrera, F.; Arias, P.; Ponce, R.; Araiza, J. Palatal zygomycosis: Experience of 21 cases. Oral Dis. 2008, 14, 569–574.
- Cruickshank, G.; Vincent, R.D.; Cherrick, H.M.; Derby, K. Rhinocerebral mucormycosis. J. Am. Dent. Assoc. 1977, 95, 1164–1168.
- Iatta, R.; Napoli, C.; Borghi, E.; Montagna, M.T. Rare mycoses of the oral cavity: A literature epidemiologic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 108, 647–655.
- Tabachnick, T.T.; Levine, B. Mucormycosis of the craniofacial structures. J. Oral. Surg. 1975, 33, 464–469.
- Eilderton, T.E. Fatal postextraction cerebral mucormycosis in an unknown diabetic. J. Oral. Surg. 1974, 32, 297–300.
- Shetty, S.R.; Punnya, V.A. Palatal mucormycosis: A rare clinical dilemma. Oral. Surg. 2008, 1, 145–148.
- Tugsel, Z.; Sezer, B.; Akalin, T. Facial swelling and palatal ulceration in a diabetic patient. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2004, 98, 630–636.
- Auluck, A. Maxillary necrosis by mucormycosis. a case report and literature review. Med. Oral. Patol. Oral. Cir. Bucal. 2007, 12, E360–E364.
- Ahmed, E.; Abou-Bakr, A.; Hussein, R.R.; El-Gawish, A.A.; Ras, A.E.; Ghalwash, D.M. Oral mucormycosis in post-COVID-19 patients: A case series. Oral Dis. 2021.
- Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P.; Edwards, J.J.; Ibrahim, A.S. Recent Advances in the Management of Mucormycosis: From Bench to Bedside. Clin. Infect. Dis. 2009, 48, 1743–1751.
- Reed, C.; Bryant, R.; Ibrahim, A.S.; Edwards JJr Filler, S.G.; Goldberg, R.; Spellberg, B. Combination polyene caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin. Infect Dis. 2008, 47, 364–371.
- McDermott, N.E.; Barrett, J.; Hipp, J.; Merino, M.J.; Lee, C.-C.; Waterman, P.; Domingo, D.L.; Walsh, T.J. Successful treatment of periodontal mucormycosis: Report of a case and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 109, e64–e69.
- Ahmad, S.R.; Ghosh, P. A Systematic Review on Mucormycosis in Corona Patients and its Treatment in India. J. Commun. Dis. 2021, 53, 236–243.
- Christenson, J.C.; Shalit, I.; Welch, D.F.; Guruswamy, A.; Marks, M.I. Synergistic action of amphotericin B and rifampin against Rhizopus species. Antimicrob. Agents Chemother. 1987, 31, 1775–1778.
- Ferguson, B.J.; Mitchell, T.G.; Moon, R.E.; Camporesi, E.M.; Farmer, J. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Clin. Infect. Dis. 1988, 10, 551–559.
- Greenberg, R.N.; Mullane, K.; van Burik, J.-A.H.; Raad, I.; Abzug, M.J.; Anstead, G.; Herbrecht, R.; Langston, A.; Marr, K.A.; Schiller, G.; et al. Posaconazole as Salvage Therapy for Zygomycosis. Antimicrob. Agents Chemother. 2006, 50, 126–133.
- León-Buitimea, A.; Garza-Cervantes, J.A.; Gallegos-Alvarado, D.Y.; Osorio-Concepción, M.; Morones-Ramírez, J.R. Nanomaterial-Based Antifungal Therapies to Combat Fungal Diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis. Pathogens 2021, 10, 1303.
- Cappellini, M.D. Iron-chelating therapy with the new oral agent ICL670 (Exjade). Best Pract. Res. Clin. Haematol. 2005, 18, 289–298.
- Boelaert, J.R.; Van Cutsem, J.; de Locht, M.; Schneider, Y.J.; Crichton, R.R. Deferoxamine augments growth and pathogenicity of Rhizopus, while hydroxypyridinone chelators have no effect. Kidney Int. 1994, 45, 667–671.
- Walsh, T.J.; Hiemenz, J.W.; Seibel, N.L.; Perfect, J.R.; Horwith, G.; Lee, L.; Silber, J.L.; DiNubile, M.J.; Reboli, A.; Bow, E.; et al. Amphotericin B lipid complex for invasive fungal infections: Analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 1998, 26, 1383–1396.
- Ibrahim, A.S.; Gebermariam, T.; Fu, Y.; Lin, L.; Husseiny, M.I.; French, S.W.; Schwartz, J.; Skory, C.D.; Edwards, J.E.; Spellberg, B.J. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Investig. 2007, 117, 2649–2657.
- Rodriȷguez, M.M.; Pastor, F.J.; Calvo, E.; Salas, V.; Sutton, D.A.; Guarro, J. Correlation of in vitro activity, serum levels, and in vivo efficacy of posaconazole against rhizopus microsporus in a murine disseminated infection. Antimicrob. Agents Chemother. 2009, 53, 5022–5025.
- Sahu, R.K.; Salem-Bekhit, M.M.; Bhattacharjee, B.; Almoshari, Y.; Ikbal, A.M.A.; Alshamrani, M.; Bharali, A.; Salawi, A.; Widyowati, R.; Alshammari, A.; et al. Mucormycosis in Indian COVID-19 Patients: Insight into Its Patho-Genesis, Clinical Manifestation, and Management Strategies. Antibiotics 2021, 10, 1079.
This entry is offline, you can click here to edit this entry!
