Fluorescein is a fluorescent organic dye used as tracer, contrasting agent or a diagnostic tool in various fields of medicine and natural sciences in general.
- fluorescein
- irradiation
- singlet oxygen
- carbon monoxide
- cytotoxicity
- metabolism
- proliferation
1. Introduction
Fluorescein is a small-molecule organic dye that exhibits strong fluorescence at neutral and alkalic pH in aqueous media. In these conditions, its main absorption peak is at 490 nm (ε490 = 76 900 M−1 cm−1) and the quantum yield of the subsequent fluorescence is 0.93[1]. It is a dark orange powder soluble in water and when dissolved in proper conditions, emitting bright green fluorescence (with maximum emission at 515 nm[2]). It belongs to a group of xanthene dyes that includes substances like eosin Y or rhodamine B. It was first synthesized by Adolf von Baeyer around 1871 by condensation of resorcinol and phthalic anhydride catalyzed with zinc chloride[3][4].
2. Application
Fluorescein is a broadly applied substance in various fields. For instance, it is commonly employed as a tracer. Some examples of this fluorescein application are utilization as a ground-water tracer[5], and use for investigating molecular movement in the bone lacunar-canalicular system[6] or for examining extravasation to study blood-spinal cord barrier permeability on animal models[7][8]. Fluorescein can be also applied as a topical fluorescent contrast agent for microendoscopy of gastrointestinal[9] or urinary tract[10] and as a pH sensitive corrosion indicator[11][12].
Moreover, it is extensively employed in ophthalmology as a diagnostic tool[13][14][15][16]. Having an important role in the diagnostics of ocular diseases, fluorescein has been included on the List of Essential Medicines, published by the World Health Organization[17]. An example of a well-established and frequently used fluorescein diagnostic technique is fluorescein angiography[13][18][19]. During diagnostic procedures, it can be administered locally[20], orally[21], or intravenously[22] with subsequent irradiation of the area of interest using blue light[13][23][24] (≈490 nm[25][26]). There are also studies that points to fluorescein’s utilization in the labeling of brain tumor tissue (high-grade gliomas) to obtain better results of its resection[27][28][29] . Fluorescein is capable of accumulating in cerebral areas with damaged blood-brain barrier[30] and therefore ensure better resection due to contrast-enhanced borderline of malignant and healthy tissue[28]. In addition, there exist studies supporting use in urology, specifically, in bladder[31][23] and penile[24] cancer surgery.
3. New discoveries
Fluorescein Irradiation Produces Biologically Active Compounds
Fluorescein itself is a relatively nontoxic substance (LD50 = 6.7 g/kg for rats[32], which is almost 20x greater than LD50 for caffeine[33] or 2x more than this value for NaCl[34]). However, it is a photoactive compound whose biological effects associated with this activity have been neglected to date. For example, fluorescein is known to photosensitize oxygen to form singlet oxygen (1O2)[35][36]. Additionally, Martinek et al.[37] and Srankova et al.[38] had shown that carbon monoxide (CO) is produced through photochemical degradation of fluorescein in a relatively high chemical yield of 40%.
Both 1O2 and CO are biologically active molecules that affect physiological processes in the human body[39][40][41]. Over the past three decades, they have also been thoroughly studied for their use in the treatment of various diseases. 1O2 is a very reactive molecule, the cytotoxic properties of which are utilized in medicine, e.g., in photodynamic therapy[42]. CO, in low concentration, acts in the body as a gasotransmitter mediating anti-inflammatory[43], antiapoptotic[44], and antiproliferative effects[45]. Although 1O2 and CO are being investigated for their potential therapeutic use in the treatment of various diseases, they both exert cytotoxicity at higher concentrations, particularly when their transport to target sites is not strictly controlled. While 1O2 causes oxidative damage and cell death[46][47][48][49], the toxicity of CO is related to its high binding affinity to blood hemoglobin[50][51][52][53] or the heme moiety of extravascular hemoproteins[54][55] such as cytochrome c oxidase[56], affecting their oxygen carrier properties or enzymatic activities, respectively. In addition, CO can trigger oxidative stress[57] and lipoperoxidation[58].
Fluorescein Irradiation Impacts Physiological Processes of HepG2 cells
Cytotoxicity of fluorescein irradiation
As has been already mentioned in this entry, fluorescein posseses relatively low toxicity. Intracellular fluorescein (administrated in the form of fluorescein diacetate (FDA) which, unlike fluorescein, is able to penetrate the cellular membrane where is hydrolyzed to fluorescein[59]) also showed minimal toxicity (Figure 1, graphs A and B). Low toxicity was also observed for its stable photoproducts (Figure 1, graphs C and D). However, simultaneous irradiation of cells treated with fluorescein led to a significant and time-dependent decrease in cell viability (Figure 1, graphs E and F), suggesting that one or more photoproducts formed during irradiation, which were not present in the solutions upon exhaustive irradiation (= stable photoproducts), were responsible for the observed cytotoxicity. This means that these species must be either volatile or short-lived (although reactive), pointing to CO and 1O2[38].
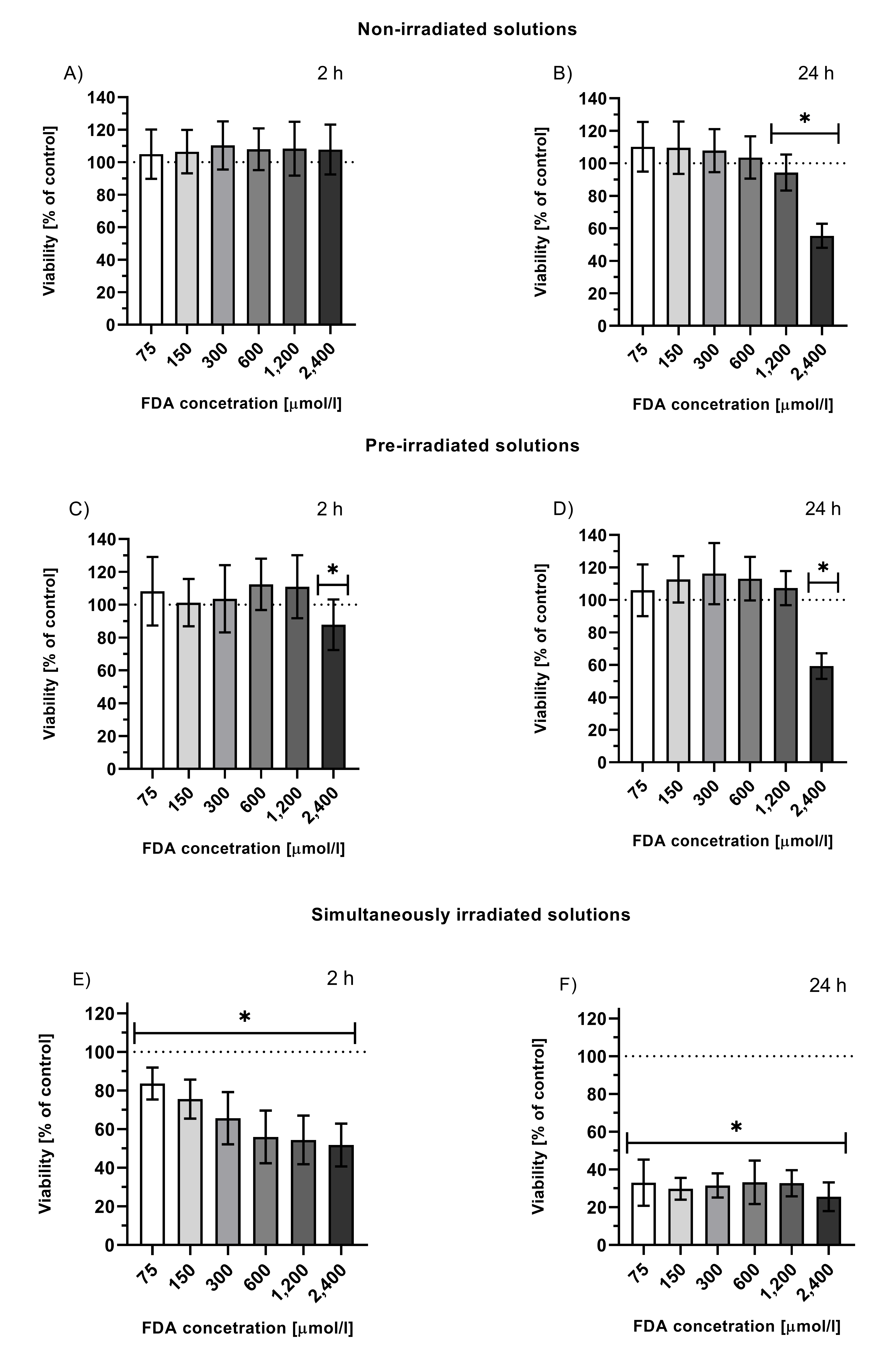
Figure 1. Viability of HepG2 cells treated with solutions of non-irradiated FDA solution (A, B), solution of fluorescein photoproducts (C, D; tir = 24 h, I = 160 mW/cm2 prior to the treatment, FDA concentration = initial concentration of FDA prior irradiation), or simultaneously irradiated solutions of FDA (E, F; irradiation throughout the entire incubation time 2 or 24 h, I = 160 mW/cm2, FDA concentration = FDA initial concentration prior irradiation); *p ≤ 0.05 vs. untreated control.
Effect on Krebs Cycle
Exposure of HepG2 cells to irradiation of intracellular fluorescein, furthermore, resulted in a significant decrease in the majority of metabolites, with the most significant changes in the concentrations of lactate, 2-hydroxyglutarate, 2-oxoglutarate, and citrate (<30% those of control)[38]. This indicates that the above-mentioned biologically active by-products of fluorescein photoexcitation might affect the overall cellular energetic metabolism.
A significant decrease in the concentrations of the Krebs cycle intermediates (2-hydroxyglutarate, glutamate, 2-oxoglutarate, and citrate) was also observed following CO treatment (enriched CO atmosphere, 100 ppm)[38], confirming the key role of this molecule in the observed attenuation of cell metabolism caused by fluorescein irradiation. These results correspond to those observed upon CO exposure that demonstrated the inhibition of respiration and glycolysis and a decrease in some Krebs cycle metabolites[60]. On the other hand, some published data have proved that CO can promote oxidative phosphorylation[61][62], mitochondrial biogenesis[63], and even an increase in cytochrome c oxidase activity[64], suggesting that the effect of CO is concentration- and tissue-dependent and reflects the overall cell/tissue status or oxygen level.
Effect on Cell Cycle
Irradiation of FDA-treated cells also resulted in a significant increase in the G0 phase and a simultaneous decrease in G2/M phase (18% decrease when compared to control) of cell cycle, indicating reduced proliferation and thus the antiproliferative and anticancer potential of fluorescein. No significant effect of CO (enriched CO atmosphere, 100 ppm) was observed on cell cycle progression, indicating no involvement of the CO released during the photoreaction in this process[38]. However, CO has been suggested to affect the cell cycle[65][66], showing that this effect might be both dose- and cell-dependent.
Comparison of Different Fluorescein Administration Modes
Treatment of cells with free fluorescein compared to treatment with FDA gave different results. Comparing the effects of these two modes of fluorescein treatment helped to assess the biological effects of 1O2 and CO when produced both intra- and extracellularly. Comparison of the cell viability indicated that when administered as a free acid, fluorescein’s negative impact on viability is significantly smaller[38]. In this case, the 1O2 molecules released during the photoreaction do not necessarily reach the intracellular compartment because of the short half-life of 1O2 (τ1/2 = 3–4 μs[67]). On the other hand, the long-lived CO (τ1/2 = 3–4 h) can freely pass through the plasma membrane and affect cellular processes when generated extracellularly, as shown by Lazarus et al.[68], who studied the intra- vs. extracellular delivery of CO using two types of CO-releasing molecules (CORMs) differing in their cellular localization. They showed that extracellular CO production exhibited a lower toxic effect on cells, whereas anti-inflammatory cell signaling processes were similar to those of intracellular delivery.
Impact of O2 Concentration on Cytotoxicity of Fluorescein Irradiation
Fluorescein irradiation cytotoxicity experiments performed in a hypoxic chamber (9% O2 level) showed that hypoxia was associated with a significantly lower drop in the viability of cells when compared to that under normoxic conditions. Three different ways of how the O2 level may influence this parameter were proposed. A lower O2 level can result in: a lower yield of 1O2 (fewer O2 molecules available for sensitization); reduced efficiency of the fluorescein photoreaction (if 1O2 is responsible for its degradation) and thus less efficient CO release; or a different cellular metabolic status, any of which ultimately affects the cell’s survival[38].
4. Conclusion and Outlook
Fluorescein is a widely used fluorescent dye that has found its application in many fields of natural sciences. Medicine is no exception, where fluorescein plays the role of a tracer, contrast compound or diagnostic agent, etc. For many of these applications is fluorescein irradiated to yield fluorescence. It was found that this irradiation results in the production of the biologically active molecules 1O2 and CO. Experiments on HepG2 cells also showed cytotoxicity, attenuation of metabolism and decreased proliferation as a result of fluorescein irradiation. This may indicate that volatile and reactive molecules produced during fluorescein photochemical reaction, from which 1O2 and CO were identified, are responsible for some adverse effects observed with fluorescein administration to patients. On the other hand, as fluorescein releases CO in substantial amounts, it might be used therapeutically as a photoCORM to release CO in target tissues. Another possibility of application is to employ both bioactive molecules, 1O2 and CO, for the therapeutic action, for example, in the treatment of cancer.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031504
References
- Robert Sjöback; Jan Nygren; Mikael Kubista; Absorption and fluorescence properties of fluorescein. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 1995, 51, L7-L21, 10.1016/0584-8539(95)01421-p.
- Monique M. Martin; Lars Lindqvist; The pH dependence of fluorescein fluorescence. Journal of Luminescence 1975, 10, 381-390, 10.1016/0022-2313(75)90003-4.
- Wei-Chuan Sun; Kyle R. Gee; And Dieter H. Klaubert; Richard P. Haugland; Synthesis of Fluorinated Fluoresceins. The Journal of Organic Chemistry 1997, 62, 6469-6475, 10.1021/jo9706178.
- von Baeyer, A.; Ueber ein neue Klasse von Farbstoffen. Berichte der Deutschen Chemischen Gesellschaft 1871, 4, 555-558, .
- David A. Sabatini; T. Ai Austin; Characteristics of Rhodamine WT and Fluorescein as Adsorbing Ground-Water Tracers. Groundwater 1991, 29, 341-349, 10.1111/j.1745-6584.1991.tb00524.x.
- Liyun Wang; Yilin Wang; Yuefeng Han; Scott C. Henderson; Robert J. Majeska; Sheldon Weinbaum; Mitchell B. Schaffler; In situ measurement of solute transport in the bone lacunar-canalicular system. Proceedings of the National Academy of Sciences 2005, 102, 11911-11916, 10.1073/pnas.0505193102.
- Dimitris N Xanthos; Isabella Püngel; Gabriele Wunderbaldinger; Jürgen Sandkühler; Effects of Peripheral Inflammation on the Blood-Spinal Cord Barrier. Molecular Pain 2012, 8, 44-44, 10.1186/1744-8069-8-44.
- Nick Cochran; Travis Rush; Susan C. Buckingham; Erik D. Roberson; The Alzheimer's disease risk factor CD2AP maintains blood–brain barrier integrity. Human Molecular Genetics 2015, 24, 6667-6674, 10.1093/hmg/ddv371.
- Sandra P. Prieto; Keith K. Lai; Jonathan A. Laryea; Jason Mizell; William C. Mustain; Timothy J. Muldoon; Fluorescein as a topical fluorescent contrast agent for quantitative microendoscopic inspection of colorectal epithelium. Biomedical Optics Express 2017, 8, 2324-2338, 10.1364/boe.8.002324.
- Timothy C. Chang; Jen-Jane Liu; Joseph C. Liao; Probe-based confocal laser endomicroscopy of the urinary tract: the technique.. Journal of Visualized Experiments 2013, , , 10.3791/4409.
- Maher A. Alodan; William H. Smyrl; Detection of Localized Corrosion Using Fluorescence Microscopy. Journal of The Electrochemical Society 1997, 144, L282-L284, 10.1149/1.1838010.
- L.M. Calle; W. Li; Microencapsulated indicators and inhibitors for corrosion detection and control. Handbook of Smart Coatings for Materials Protection 2014, , , 10.1533/9780857096883.2.370.
- Rosario Brancato; Francesco Bandello; Rosangela Lattanzio; Iris fluorescein angiography in clinical practice. Survey of Ophthalmology 1997, 42, 41-70, 10.1016/s0039-6257(97)84042-8.
- FRCS Caroline J MacEwan; Frcs James D H Young; The Fluorescein Disappearance Test (FDT): An Evaluation of Its Use in Infants. Journal of Pediatric Ophthalmology & Strabismus 1991, 28, 302-305, 10.3928/0191-3913-19911101-04.
- Revelle Littlewood; Susan P Mollan; Irene M Pepper; Simon J Hickman; The Utility of Fundus Fluorescein Angiography in Neuro-Ophthalmology. Neuro-Ophthalmology 2019, 43, 217-234, 10.1080/01658107.2019.1604764.
- Daniel Sibley; Daniel F. P. Larkin; Update on Herpes simplex keratitis management. Eye 2020, 34, 2219-2226, 10.1038/s41433-020-01153-x.
- 22nd Model List of Essential Medicines . World Health Organization. Retrieved 2022-4-21
- Maurice F. Rabb; Thomas C. Burton; Howard Schatz; Lawrence A. Yannuzzi; Fluorescein angiography of the fundus: A schematic approach to interpretation. Survey of Ophthalmology 1978, 22, 387-403, 10.1016/0039-6257(78)90134-0.
- Alberto La Mantia; Rengin A. Kurt; Samantha Mejor; Catherine Egan; Adnan Tufail; Pearse A. Keane; Dawn A. Sim; COMPARING FUNDUS FLUORESCEIN ANGIOGRAPHY AND SWEPT-SOURCE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY IN THE EVALUATION OF DIABETIC MACULAR PERFUSION. Retina 2019, 39, 926-937, 10.1097/iae.0000000000002045.
- British National Formulary 81 . Web of Pharma. Retrieved 2022-4-21
- Tsutomu Hara; Mikino Inami; Takako Hara; Efficacy and safety of fluorescein angiography with orally administered sodium fluorescein. American Journal of Ophthalmology 1998, 126, 560-564, 10.1016/s0002-9394(98)00112-3.
- Harold R. Novotny; David L. Alvis; A Method of Photographing Fluorescence in Circulating Blood in the Human Retina. Circulation 1961, 24, 82-86, 10.1161/01.cir.24.1.82.
- Geoffrey A. Sonn; Sha-Nita E. Jones; Tatum V. Tarin; Christine B. Du; Kathleen E. Mach; Kristin C. Jensen; Joseph C. Liao; Optical Biopsy of Human Bladder Neoplasia With In Vivo Confocal Laser Endomicroscopy. Journal of Urology 2009, 182, 1299-1305, 10.1016/j.juro.2009.06.039.
- Eugene Shkolyar; Mark A. Laurie; Kathleen E. Mach; Dharati R. Trivedi; Dimitar V. Zlatev; Timothy C. Chang; Thomas J. Metzner; John T. Leppert; Chia-Sui Kao; Joseph C. Liao; et al. Optical biopsy of penile cancer with in vivo confocal laser endomicroscopy. Urologic Oncology: Seminars and Original Investigations 2019, 37, 809.e1-809.e8, 10.1016/j.urolonc.2019.08.018.
- Oliver R. Marmoy; Robert H. Henderson; Kuan Ooi; Recommended protocol for performing oral fundus fluorescein angiography (FFA) in children. Eye 2020, 36, 234-236, 10.1038/s41433-020-01328-6.
- Daniel X. Hammer; R. Daniel Ferguson; Ankit H. Patel; Vanessa Vazquez; Deeba Husain; Angiography with a multifunctional line scanning ophthalmoscope. Journal of Biomedical Optics 2012, 17, 0260081-02600811, 10.1117/1.jbo.17.2.026008.
- Linda M. Wang; Matei A. Banu; Peter Canoll; Jeffrey N. Bruce; Rationale and Clinical Implications of Fluorescein-Guided Supramarginal Resection in Newly Diagnosed High-Grade Glioma. Frontiers in Oncology 2021, 11, -, 10.3389/fonc.2021.666734.
- Francesco Acerbi; Morgan Broggi; Marica Eoli; Elena Anghileri; Claudio Cavallo; Carlo Boffano; Roberto Cordella; Lucia Cuppini; Bianca Pollo; Marco Schiariti; et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas?. Neurosurgical Focus 2014, 36, E5, 10.3171/2013.11.focus13487.
- Francesco Acerbi; Claudio Cavallo; Morgan Broggi; Roberto Cordella; Elena Anghileri; Marica Eoli; Marco Schiariti; Giovanni Broggi; Paolo Ferroli; Fluorescein-guided surgery for malignant gliomas: a review. Neurosurgical Review 2014, 37, 547-557, 10.1007/s10143-014-0546-6.
- Oskar Steinwall; Igor Klatzo; Selective Vulnerability of the Blood-Brain Barrier in Chemically Induced Lesions*. Journal of Neuropathology & Experimental Neurology 1966, 25, 542-559, 10.1097/00005072-196610000-00004.
- Philippe E. Zimmern; David Laub; Gary E. Leach; Fluorescein Angiography of the Bladder: Technique and Relevance to Bladder Cancer and Interstitial Cystitis Patients. Journal of Urology 1995, 154, 62-65, 10.1016/s0022-5347(01)67225-2.
- Samuel L. Yankell; Joseph J. Loux; Acute Toxicity Testing of Erythrosine and Sodium Fluorescein in Mice and Rats. Journal of Periodontology 1977, 48, 228-231, 10.1902/jop.1977.48.4.228.
- Richard H. Adamson; The acute lethal dose 50 (LD50) of caffeine in albino rats. Regulatory Toxicology and Pharmacology 2016, 80, 274-276, 10.1016/j.yrtph.2016.07.011.
- E Walum; Acute oral toxicity.. Environmental Health Perspectives 1998, 106, 497-503, 10.1289/ehp.98106497.
- Yoshiharu Usui; DETERMINATION OF QUANTUM YIELD OF SINGLET OXYGEN FORMATION BY PHOTOSENSITIZATION. Chemistry Letters 1973, 2, 743-744, 10.1246/cl.1973.743.
- E. Gandin; Y. Lion; A. Van de Vorst; QUANTUM YIELD OF SINGLET OXYGEN PRODUCTION BY XANTHENE DERIVATIVES. Photochemistry and Photobiology 1983, 37, 271-278, 10.1111/j.1751-1097.1983.tb04472.x.
- Marek Martínek; Lucie Ludvíková; Mária Šranková; Rafael Navrátil; Lucie Muchová; Jiří Huzlík; Libor Vítek; Petr Klán; Peter Šebej; Photochemistry of Common Xanthene Fluorescent Dyes as Efficient Visible-light Activatable CO-Releasing Molecules. null 2022, , , 10.26434/chemrxiv-2022-pn3x0.
- Mária Šranková; Aleš Dvořák; Marek Martínek; Peter Šebej; Petr Klán; Libor Vítek; Lucie Muchová; Antiproliferative and Cytotoxic Activities of Fluorescein—A Diagnostic Angiography Dye. International Journal of Molecular Sciences 2022, 23, 1504, 10.3390/ijms23031504.
- Stefan W. Ryter; Leo E. Otterbein; Carbon monoxide in biology and medicine. BioEssays 2004, 26, 270-280, 10.1002/bies.20005.
- Briviba, K.; Klotz, L.-O.; Sies, H.; Toxic and signaling effects of photochemically or chemically generated singlet oxygen in biological systems. Biol. Chem. 1997, 378,, 1259–1265, .
- Devasagayam, T.; Kamat, J.P.; Biological significance of singlet oxygen. Indian J. Exp. Biol. 2002, 40, 680–692, .
- Patrizia Agostinis; Kristian Berg; Keith A. Cengel Md; Thomas H. Foster; Albert W. Girotti; Sandra O. Gollnick; Stephen M. Hahn Md; Michael R. Hamblin; Asta Juzeniene; David Kessel; et al. Photodynamic therapy of cancer: An update. CA: A Cancer Journal for Clinicians 2011, 61, 250-281, 10.3322/caac.20114.
- Leo E. Otterbein; Fritz H. Bach; Jawed Alam; Miguel Soares; Hong Tao Lu; Mark Allen Wysk; Roger J. Davis; Richard A. Flavell; Augustine M. K. Choi; Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Medicine 2000, 6, 422-428, 10.1038/74680.
- Sophie Brouard; Leo E. Otterbein; Josef Anrather; Edda Tobiasch; Fritz H. Bach; Augustine M.K. Choi; Miguel Soares; Carbon Monoxide Generated by Heme Oxygenase 1 Suppresses Endothelial Cell Apoptosis. Journal of Experimental Medicine 2000, 192, 1015-1026, 10.1084/jem.192.7.1015.
- Toshisuke Morita; S. Alex Mitsialis; Hideo Koike; Yuxiang Liu; Stella Kourembanas; Carbon Monoxide Controls the Proliferation of Hypoxic Vascular Smooth Muscle Cells. Journal of Biological Chemistry 1997, 272, 32804-32809, 10.1074/jbc.272.52.32804.
- M L Agarwal; M E Clay; E J Harvey; H H Evans; A R Antunez; N L Oleinick; Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells.. Cancer Research 1991, 51, 5993-6, .
- W M Star; H P Marijnissen; A E Van Den Berg-Blok; J A Versteeg; K A Franken; H S Reinhold; Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers.. Cancer Research 1986, 46, -, .
- Norman I. Krinsky; Singlet oxygen in biological systems. Trends in Biochemical Sciences 1977, 2, 35-38, 10.1016/0968-0004(77)90253-5.
- Michael J. Davies; Singlet oxygen-mediated damage to proteins and its consequences. Biochemical and Biophysical Research Communications 2003, 305, 761-770, 10.1016/s0006-291x(03)00817-9.
- Ivan Blumenthal; Carbon Monoxide Poisoning. Journal of the Royal Society of Medicine 2001, 94, 270-272, 10.1177/014107680109400604.
- C. G. Douglas; J. S. Haldane; The laws of combination of haemoglobin with carbon monoxide and oxygen. The Journal of Physiology 1912, 44, 275-304, 10.1113/jphysiol.1912.sp001517.
- Bernard, C. . Leçons sur Les Effets des Substances Toxiques et Médicamenteuses; Librairie, J.B., Ed.; Baillière et Fils: Leon, France, 1857; pp. -.
- John Haldane; J. Lorrain Smith; The Absorption of Oxygen by the Lungs. The Journal of Physiology 1897, 22, 231-258, 10.1113/jphysiol.1897.sp000689.
- Norman Nomof; James Hopper; Ellen Brown; Kenneth Scott; Reidar Wennesland; Simultaneous Determinations of the Total Volume of Red Blood Cells by Use of Carbon Monoxide and Chromium51 IN HEALTHY AND DISEASED HUMAN SUBJECTS1. Journal of Clinical Investigation 1954, 33, 1382-1387, 10.1172/jci103015.
- Ronald F. Coburn; THE CARBON MONOXIDE BODY STORES. Annals of the New York Academy of Sciences 1970, 174, 11-22, 10.1111/j.1749-6632.1970.tb49768.x.
- Òscar Miró; Jordi Casademont; Antoni Barrientos; Álvaro Urbano-Márquez; Francesc Cardellach; Mitochondrial Cytochrome c Oxidase Inhibition during Acute Carbon Monoxide Poisoning. Pharmacology & Toxicology 1998, 82, 199-202, 10.1111/j.1600-0773.1998.tb01425.x.
- J Zhang; C A Piantadosi; Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain.. Journal of Clinical Investigation 1992, 90, 1193-1199, 10.1172/jci115980.
- S. R. Thom; Carbon monoxide-mediated brain lipid peroxidation in the rat. Journal of Applied Physiology 1990, 68, 997-1003, 10.1152/jappl.1990.68.3.997.
- B Rotman; B W Papermaster; Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters.. Proceedings of the National Academy of Sciences 1966, 55, 134-141, 10.1073/pnas.55.1.134.
- Patrycja Kaczara; Barbara Sitek; Kamil Przyborowski; Anna Kurpinska; Kamil Kus; Marta Stojak; Stefan Chlopicki; Antiplatelet Effect of Carbon Monoxide Is Mediated by NAD + and ATP Depletion. Arteriosclerosis, Thrombosis, and Vascular Biology 2020, 40, 2376-2390, 10.1161/atvbaha.120.314284.
- Marialuisa Lavitrano; Ryszard T. Smolenski; Antonino Musumeci; Massimo Maccherini; Ewa Slominska; Ernesto Florio; Adele Bracco; Antonio Mancini; Giorgio Stassi; Mariella Patti; et al. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. The FASEB Journal 2004, 18, 1093-1095, 10.1096/fj.03-0996fje.
- Tung-Yu Tsui; Yeung-Tung Siu; Hans J. Schlitt; Sheung-Tat Fan; Heme oxygenase-1-derived carbon monoxide stimulates adenosine triphosphate generation in human hepatocyte. Biochemical and Biophysical Research Communications 2005, 336, 898-902, 10.1016/j.bbrc.2005.08.187.
- Hagit B. Suliman; Martha S. Carraway; Lynn G. Tatro; Claude A. Piantadosi; A new activating role for CO in cardiac mitochondrial biogenesis. Journal of Cell Science 2007, 120, 299-308, 10.1242/jcs.03318.
- Cláudia Sf Queiroga; Ana S Almeida; Paula M Alves; Catherine Brenner; Helena LA Vieira; Carbon monoxide prevents hepatic mitochondrial membrane permeabilization. BMC Cell Biology 2011, 12, 10-10, 10.1186/1471-2121-12-10.
- Ruiping Song; Raja S. Mahidhara; Fang Liu; Wen Ning; Leo E. Otterbein; Augustine M. K. Choi; Carbon Monoxide Inhibits Human Airway Smooth Muscle Cell Proliferation via Mitogen-Activated Protein Kinase Pathway. American Journal of Respiratory Cell and Molecular Biology 2002, 27, 603-610, 10.1165/rcmb.4851.
- Hyun-Ock Pae; Gi-Su Oh; Byung-Min Choi; Soo-Cheon Chae; Young-Myeong Kim; Khee-Rhin Chung; Hun-Taeg Chung; Carbon Monoxide Produced by Heme Oxygenase-1 Suppresses T Cell Proliferation via Inhibition of IL-2 Production. The Journal of Immunology 2004, 172, 4744-4751, 10.4049/jimmunol.172.8.4744.
- Francis Wilkinson; W. Phillip Helman; Alberta B. Ross; Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. An Expanded and Revised Compilation. Journal of Physical and Chemical Reference Data 1995, 24, 663-677, 10.1063/1.555965.
- Livia S. Lazarus; Casey Simons; Ashley Arcidiacono; Abby Benninghoff; Lisa M. Berreau; Extracellular vs Intracellular Delivery of CO: Does It Matter for a Stable, Diffusible Gasotransmitter?. Journal of Medicinal Chemistry 2019, 62, 9990-9995, 10.1021/acs.jmedchem.9b01254.
- Oskar Steinwall; Igor Klatzo; Selective Vulnerability of the Blood-Brain Barrier in Chemically Induced Lesions*. Journal of Neuropathology & Experimental Neurology 1966, 25, 542-559, 10.1097/00005072-196610000-00004.
- Richard H. Adamson; The acute lethal dose 50 (LD50) of caffeine in albino rats. Regulatory Toxicology and Pharmacology 2016, 80, 274-276, 10.1016/j.yrtph.2016.07.011.
- E Walum; Acute oral toxicity.. Environmental Health Perspectives 1998, 106, 497-503, 10.1289/ehp.98106497.
