Given the physiological toll that CRS/HIPEC extracts and the potential for adverse outcomes, various risk-stratification tools have been developed to determine which patients with PC will derive benefit from this intervention. Those techniques demonstrating efficacy for appendiceal and CRCs are described.
As with most cancers, complete tumor resection has been shown to improve outcomes for patients with PC. Two systems defining surgical margins are commonly used in clinical practice and throughout the literature. Neither has been found to be superior.
6.1. HIPEC with Curative Intent
A landmark randomized controlled trial (RCT) evaluating CRS/HIPEC was published in 2003 [
124]. Verwaal et al. compared standard-of-care systemic chemotherapy (n = 51) to CRS/HIPEC (n = 54) in patients with PC from appendiceal or CRC. Systemic therapy included 5-FU + leucovorin for 26 weeks or irinotecan for 6 months (if patients had received 5-FU treatment within the previous 12 months). Patients in the chemotherapy group underwent surgery for intestinal obstruction with diversion with bypass or stoma. CRS/HIPEC patients received mitomycin C (MMC) for 90 min and standard adjuvant systemic therapy. Complete cytoreduction was achieved in 41% of patients. Compared to control, patients who underwent CRS/HIPEC demonstrated an overall survival (OS) of 22.2 months vs. 12.6 months in the chemotherapy arm. Surgical mortality associated with CRS/HIPEC was 8%, which was consistent with contemporary studies. However, overall mortality was equal between the two cohorts for the first 6 months. Although this study was the first to definitively demonstrate a survival benefit favoring CRS/HIPEC, there were substantial limitations. Notably, patients with extensive PC were included in both cohorts. The study noted that these patients had poorer outcomes regardless of treatment, giving one of the first reports that disease burden could predict long-term outcomes from CRS/HIPEC. Furthermore, only a minority of patients in the CRS/HIPEC arm had complete cytoreduction. A subanalysis suggested that achieving complete cytoreduction was required for improved outcomes, supporting data that showed a complete macroscopic eradication of disease is associated with optimal outcomes. In 2008, long-term results from the study were released and continued to show that patients with complete cytoreduction maintained a survival benefit. In fact, the 5-year survival was 45% with complete cytoreduction but only 8–10% for incomplete cytoreduction or systemic therapy alone, demonstrating that surgical intervention resulting in incomplete cytoreduction offered no survival benefit over systemic therapies [
125].
A more recent multicenter RCT released in 2021, PRODIGE 7, did not show a benefit of CRS/HIPEC using high-dose oxaliplatin for 30 min over CRS alone [
126]. Although this study demonstrated a substantial improvement in overall survival, over 40 months in both groups, this study had many shortcomings that mandate a cautious interpretation of these study results. First, the study did not meet its primary endpoint and was not powered for hypothesis testing. Notably, the study included patients who received incomplete cytoreduction with CC-1 resections, which is an established factor that directly impacts survival. In the control group, 12% of patients crossed-over to receive subsequent CRS/HIPEC due to isolated peritoneal recurrence. However, no patients in the CRS/HIPEC group received secondary CRS/HIPEC. These differences are difficult to control with the intent-to-treat analysis used in this study.
Additionally, the majority of patients in the study were heavily pre-treated with oxaliplatin-based therapies, which may have resulted in peritoneal tumors resistant to oxaliplatin-based HIPEC, thus resulting in less effective disease control from HIPEC therapy [
127]. Moreover, the carrier fluid (D5W), dosage of oxaliplatin (360–460 mg/m
2), and lack of concomitant IV 5-FU during HIPEC call into question the efficacy of the HIPEC treatment in this study, which does not follow current US guidelines (
Table 1). Furthermore, the adjuvant systemic therapy used in this study consisted only of 5-FU and leucovorin, which is inconsistent with current US standards-of-care [
128]. A lack of adherence to current US treatment guidelines limits the applicability of these study results to the US patient population. Additionally, patients with high PCI scores (20–25) were included despite lower PCI scores previously demonstrating an enhanced benefit from HIPEC. In fact, subgroup analyses showed that patients with PCI scores between 11–15 had improved survival. However, because 25% of the patients included in the study had a PCI > 15, the overall survival findings remain unclear. Additional factors raise questions about the accuracy and validity of this study. Patients in the HIPEC intervention arm experienced more post-operative complications compared to the control arm: 42% vs. 32% at 30 days and 26% vs. 15% at 60 days, respectively. Furthermore, the mortality rates were 3% for the HIPEC group and 2% for the control group, both above the established average of <1% for high-volume CRS/HIPEC centers, such as those included here. Altogether, these limitations create an unclear picture requiring restraint when interpreting these study results.
Table 1. Recommended HIPEC Regimens for Appendiceal and Colorectal Cancers in the US. The most common established regimens across societies are shown and first-line recommendations are bolded. MMC (mitomycin C), PCI (peritoneal carcinomatosis index).
6.2. HIPEC for Palliation
PC recurrence following CRS/HIPEC is common, even with CC-0 resections, particularly in patients with high PCI scores and unfavorable histologies. In fact, up to 46% of colorectal and 100% of perforated appendiceal cancers may develop recurrent peritoneal disease despite multimodal therapies [
133,
134,
135]. In conditions where CRS/HIPEC is not expected to be curative, such as those with PCI > 19 and CRC with ascites, CRS/HIPEC can provide palliation for debilitating symptoms and improve quality of life. Multiple retrospective studies have shown that HIPEC can successfully control malignant ascites in up to 95% of patients with underlying GI malignancies [
83,
84,
136,
137,
138,
139,
140]. Furthermore, because complete cytoreduction is not feasible in these patients, laparoscopic HIPEC is a reasonable approach with less associated morbidity [
83,
137,
138,
139,
140,
141,
142]. Indeed, the Chicago Consensus Working Group now recommends laparoscopic HIPEC for the management of malignant ascites in patients who are not candidates for curative-intent CRS/HIPEC [
143].
6.3. HIPEC for Prevention of Disease Recurrence
In patients at risk of developing PC, two proactive approaches have been proposed: (1) prophylactic HIPEC at the time of primary tumor resection and (2) second-look surgery including HIPEC. The COLOPEC trial published in 2019 investigated prophylactic HIPEC in patients undergoing oncologic resection of T4 or perforated CRC without PC [
144]. The PROPHYLOCHIP (PRODIGE 15) trial evaluated the use of HIPEC during routine second-look surgery after complete CRS in patients with CRC [
145]. However, there were significant limitations with both of these studies, and the lack of benefit is contested because these studies also suggest a potential benefit from HIPEC in the setting of macroscopic disease. However, like the PRODIGE 7 trial, both studies had design flaws limiting the applicability of their results.
The COLOPEC trial was confounded by the fact that 9% of patients in the prophylactic HIPEC arm were found to have PC shortly after surgery, indicating synchronous metastasis that were missed during surgery. These patients did not receive prophylactic HIPEC or complete CRS prior to HIPEC, which is a major contributing factor to survival, as discussed previously. Furthermore, patients in this study had HIPEC at the time of traditional oncologic resection (up to 10 days after surgery) or 5–8 weeks after surgery, thereby introducing confounding effects based on the timing of HIPEC. Additionally, similar to the PRODIGE 7 trial, the utilized HIPEC regimen was 460 mg/m2 oxaliplatin for 30 min, which differs from the standard US HIPEC recommendations (Table 1). Although IV 5-FU and leucovorin were administered concomitantly with intraperitoneal oxaliplatin, similar to US protocols with oxaliplatin-based HIPEC, this regimen is not the first-line standard-of-care HIPEC procedure in the US and it results in data that are not directly applicable to the US population.
In the PROPHYLOCHIP trial, patients with PC from CRC underwent traditional oncologic resection plus CRS. However, contrary to common standard practice, HIPEC was not performed in these patients following CRS during the initial surgery. In the investigative arm, second-look surgery was performed after the completion of 6 months of standard systemic chemotherapy. HIPEC regimens included: 460 mg/m2 oxaliplatin for 30 min with IV 400 mg/m2 5-FU, 300 mg/m2 oxaliplatin + 200 mg/m2 irinotecan for 30 min with 400 mg/m2 5-FU IV, or 35 mg/m2 MMC for 30 min for patients with previous neurotoxicity from oxaliplatin. The control arm included adjuvant systemic therapy alone. Again, as with the PRODIGE 7 trial, the use of non-standard and oxaliplatin-based HIPEC regimens in the majority of patients calls into question the efficacy of HIPEC performed in this study. Although peritoneal disease recurrence was lower in the investigational arm of this study, perhaps due to unintentional under-staging by utilizing preoperative imaging alone and, therefore, a higher portion of patients with an intermediate risk of PC recurrence, the 3-year disease-free survival was not significantly different.
6.4. Alternative Intraperitoneal Chemotherapy Techniques
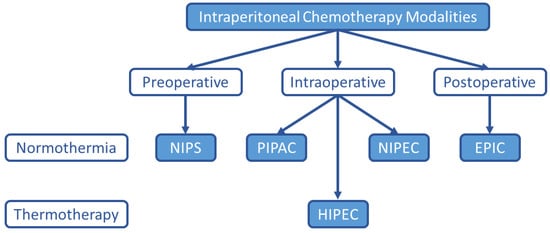
Although HIPEC remains the most common intraperitoneal chemotherapy treatment option for PC, many alternatives have been explored and are under investigation as listed in Figure 1.
Figure 1. Alternate Intraperitoneal Chemotherapy Treatment Modalities. Although HIPEC is the current standard-of-care intraperitoneal chemotherapy treatment option for PC from appendiceal and colorectal malignancies, other intraperitoneal chemotherapy treatment strategies are under investigation. NIPS (neoadjuvant intraperitoneal and systemic chemotherapy), PIPAC (pressurized intraperitoneal aerosol chemotherapy), HIPEC (hyperthermic intraperitoneal chemotherapy), NIPEC (normothermic intraperitoneal chemotherapy), EPIC (early postoperative intraperitoneal chemotherapy).
6.4.1. Early Postoperative Intraperitoneal Chemotherapy (EPIC)
In 1990, Sugarbaker initially described EPIC, which included intraperitoneal chemotherapy administered on postoperative days 1 and 5 following CRS [
146]. In an RCT published in 2016, Cashin and colleagues found that the effect of CRS/EPIC was superior to systemic chemotherapy alone for CRC with PC [
147]. However, more recent studies evaluating the utility of adding EPIC to CRS/HIPEC regimens show increased morbidity and surgical complications compared to CRS/HIPEC without EPIC, and survival benefits are unclear between the two regimens [
148,
149,
150,
151]. Concerns of increased perioperative morbidity, the development of increased postoperative adhesions, an incomplete distribution of chemotherapy throughout the abdomen and pelvis in the perioperative setting, and unanswered questions surrounding EPIC efficacy compared to HIPEC have resulted in EPIC falling out of favor [
146,
152,
153,
154]. The ICARuS study is an important ongoing phase 2 RCT that will directly compare CRS/HIPEC vs. CRS/EPIC and will hopefully answer questions regarding which method offers superior efficacy (NCT01815359). Additionally, for difficult-to-treat cancers with poor responses to current systemic therapy and CRS/HIPEC regimens, long-term EPIC regimens have demonstrated longer-term benefits with additional studies underway (NCT05056389) [
155,
156].
6.4.2. Normothermic Intraperitoneal Chemotherapy (NIPEC)
Given continued high PC recurrence rates following current CRS/HIPEC regimens, NIPEC has been proposed in an effort to enable the utilization of non-thermostable chemotherapies that may result in improved efficacy and outcomes [
130,
157]. However, few trials have directly investigated the efficacy of intraoperative NIPEC vs. standard HIPEC regimens. A 2001 RCT by Yonemura et al. demonstrated that the synergistic cytotoxicity observed with combined hyperthermia and MMC provided a significantly better 5-year survival compared to NIPEC for patients with gastric cancer (61% vs. 44%) [
158]. However, a meta-analysis in 2012 showed no benefit of NIPEC over HIPEC for gastric cancer [
159]. A study comparing HIPEC to NIPEC is underway for ovarian cancer [
160]. Historical and future prospective studies evaluating NIPEC for other cancer types, including appendiceal and CRC, are lacking.
6.4.3. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
First proposed in 2011, PIPAC takes advantage of the increased intra-abdominal pressure created during laparoscopy to deliver aerosolized particles to residual tumor cells, which promotes tissue penetration and, thereby, the efficacy of intraperitoneal chemotherapy [
161,
162]. This principle has been theorized to overcome the chemoresistance observed with some HIPEC agents [
163]. Further studies have established favorable pharmacokinetic and safety profiles, efficacy for isolated refractory PC, and ease of repeated administration [
161,
162,
164,
165,
166,
167,
168]. However, few studies have directly compared HIPEC to PIPAC. One recent pharmacokinetic study demonstrated that PIPAC and HIPEC resulted in similar systemic and parietal peritoneal absorption, but visceral peritoneal absorption was significantly higher in the PIPAC group, suggesting improved penetration of chemotherapy into tissues [
169]. Pre-clinical studies have suggested a combined HIPEC + PIPAC technique may take advantage of synergistic cytotoxicity and improved tumor penetration with hyperthermia during PIPAC [
170]. Further studies are required to evaluate the efficacy of HIPEC compared to PIPAC.
6.4.4. Neoadjuvant Intraperitoneal and Systemic Chemotherapy (NIPS)
Combined with systemic neoadjuvant therapies, neoadjuvant intraperitoneal chemotherapy aims to reduce the overall peritoneal tumor burden to make CRS feasible and improve rates of complete cytoreduction [
167]. For ovarian, gastric, and CRCs, NIPS has shown low morbidity and mortality and indeed shown higher rates of complete cytoreduction [
167,
171,
172]. Yonemura and colleges described the survival benefit of using NIPS for gastric cancer [
173,
174]. Conversely, the PHEONIX trial failed to demonstrate the superiority of NIPS vs. systemic therapy alone for gastric cancer [
175]. However, this trial was underpowered for statistical results and enrolled more patients with ascites, a known poor prognostic factor, in the NIPS intervention group. Additionally, a subanalysis revealed that neoadjuvant intraperitoneal chemotherapy without systemic IV chemotherapy may offer survival benefits. Since then, retrospective studies have suggested the superiority of NIPS over systemic therapy alone [
171,
176,
177]. Additional studies evaluating NIPS with non-traditional systemic chemotherapies are underway [
177]. Survival outcomes following NIPS remain mixed for cancers other than gastric, however, and require further prospective studies [
167,
178]. One upcoming trial aims to evaluate the effect of neoadjuvant systemic chemotherapy and PIPAC prior to CRS/HIPEC for CRC (NCT04475159). The Dragon II trial aims to evaluate NIPS using laparoscopic HIPEC prior to curative-intent CRS/HIPEC for gastric cancer [
179]. These studies will help to determine if neoadjuvant intraperitoneal chemotherapy should be incorporated into future recommendations.
6.5. HIPEC Agents and Adjunctive Therapies
6.5.1. Current Regimens
Ideal HIPEC agents have a large molecular weight, demonstrate stability and synergistic effects with heat, have a large peritoneal to plasma area-under-the-curve (AUC) ratio demonstrating limited systemic toxicity, and show antitumor efficacy against the tumor of interest [
130]. Commonly used HIPEC agents meeting these criteria include MMC, cisplatin, oxaliplatin, paclitaxel, doxorubicin, and 5-FU [
128,
130]. However, the numerous aforementioned requirements have limited the development and evaluation of new HIPEC therapies, resulting in little evolution in HIPEC regimens for decades. MMC, the most commonly used agent, was discovered in the 1950s and has been in use with HIPEC since the 1980s [
180]. In 2002, Elias et al. described bidirectional HIPEC with concomitant IV 5-FU to potentiate the cytotoxicity of oxaliplatin, the second most common HIPEC agent, by targeting tumors from the visceral and parietal sides [
181]. These results were supported by additional groups and changed the standard recommendations for oxaliplatin-based HIPEC [
182].
Since that time, however, there have been little to no changes to HIPEC regimens, resulting in different recommendations worldwide. For instance, for appendiceal and CRC-derived PC, MMC is preferred over oxaliplatin-containing regimens in the US, while oxaliplatin regimens are favored in Europe, which is reflected in how historical and emerging HIPEC studies are developed and carried out in each region [
183]. The multitude of alternative HIPEC regimens has resulted in clinical studies with fundamentally different designs that limit the applicability of results to patients in other regions [
183]. Of the limited data available, one RCT in mucinous appendiceal cancer demonstrated that patients with baseline thrombocytopenia may benefit from MMC, while those with baseline leukopenia may benefit from oxaliplatin [
184]. Neither agent was found to have superior outcomes, however.
Additional retrospective analyses have shown mixed results when evaluating the efficacy and safety profiles between these commonly used agents when treating CRC, but more studies demonstrate equivalent efficacy [
185,
186,
187,
188,
189,
190,
191,
192,
193,
194,
195,
196]. Further studies comparing oxaliplatin vs. MMC + doxorubicin [
197], irinotecan vs. oxaliplatin [
198], oxaliplatin ± irinotecan [
181,
199], and MMC vs. melphalan [
200] have been performed but have not yielded a definitively superior regimen. Upcoming trials directly comparing commonly used HIPEC regimens include: oxaliplatin ± irinotecan (NCT04861558), oxaliplatin vs. oxaliplatin + 5FU + irinotecan (NCT04861558), oxaliplatin vs. MMC vs. cisplatin + doxorubicin vs. cisplatin + MMC (NCT04847063). Despite these necessary forthcoming studies, comparisons of HIPEC agent doses are also lacking. Intra-regional regimen preferences between weight-based and flat dose regimens are common (
Table 1). To this end, our institution is preparing to study the safety and efficacy of different commonly used MMC dosages used during HIPEC for appendiceal and CRC (NCT04779554). Altogether, the results from these essential trials will help to establish the most efficacious regimen(s) and aid in the development and adoption of standard international HIPEC recommendations.
6.5.2. Emerging Therapeutics
Given the high frequency of PC recurrence, novel agents are required to make HIPEC more effective and establish curative treatment for those cancers that continue to harbor poor outcomes. New cytotoxic HIPEC agents under evaluation include MOC31PE [
201,
202], miRNA-409-3p [
203], nanoparticle pegylated liposomal doxorubicin [
204], Radspherin
® (NCT03732781), raltitrexed (NCT04761185), and lobaplatin (NCT04845490 and NCT04808466) [
205,
206]. Additionally, our group recently completed a phase 1 study evaluating the safety of nano-liposomal irinotecan (nal-Iri) during HIPEC for appendiceal and CRC (NCT04088786). Nal-Iri fulfills all requirements for optimal HIPEC agents, including thermostability, large molecular weight, low systemic absorption, and activity against CRC. CRC systemic therapy regimens including nal-Iri have demonstrated improved efficacy against chemo-resistant tumors, including traditional irinotecan [
207]. The results from our dose escalation study (70–280 mg/m
2) will lay the groundwork for upcoming phase 2 studies to evaluate nal-Iri efficacy in HIPEC for patients with appendiceal and CRCs.
The modern rise of novel immuno-oncologic therapies (IO) has impacted the treatment of many cancers, and PC is on the beginning path of these discoveries. CRS has itself been proposed to be a form of immunotherapy [
208]. Subsequent studies have confirmed that tumor resection can re-activate the native immune system to an anti-tumor phenotype [
209,
210,
211]. In fact, catumaxomab, a trifunctional antibody, was approved in Europe for intraperitoneal use to treat malignant ascites, has demonstrated promise to directly treat GI-derived PC, and is undergoing additional evaluation of efficacy for curative intent (NCT04222114, NCT01815528, and NCT01246440) [
212,
213,
214,
215,
216]. Consequently, interest in intraperitoneal IOs has grown rapidly in recent years [
217,
218,
219]. Upcoming and ongoing trials evaluating IOs as alternative intraperitoneal agents include: immune checkpoint inhibitors (ICIs), such as nivolumab ± ipilimumab (NCT03959761, NCT03508570, and NCT03172416), pembrolizumab (NCT03734692), and IMP321 (NCT03252938); TLR-3 agonists (NCT03734692); CAR-T and cytotoxic T-cell infusions (NCT03682744, NCT03323944, NCT03563326, NCT03907527, NCT02498912, and NCT03735589), IL-2 ± NK cell infusions (NCT04630769, NCT02976142, NCT02118285, and NCT03213964); immune modulators (NCT02219893); oncolytic viruses (NCT02759588 and NCT03663712); and personalized neoantigen vaccines (NCT03715985). Trials evaluating adjunctive IOs are also numerous and include the ICI camrelizumab (NCT04889768).
7. Summary of Recommendations and Conclusions
CRS/HIPEC has demonstrated substantial survival benefits for select patients with PC from appendiceal and CRCs (PCI < 20). These procedures should be performed at high-volume, multidisciplinary expert centers for optimal outcomes. Although CRS has undergone near universal standardization, HIPEC regimens remain diverse. Even recent consensus guidelines include various intraperitoneal chemotherapy regimens based on flat or weight-based dosing. Prospective studies supporting these regimens remain limited, and recent RCTs have produced contentious results. Carefully designed RCTs comparing current standard-of-care HIPEC regimens are needed to definitively identify superior regimens and are underway. Additional studies evaluating novel HIPEC agents, in particular IOs, are also desperately needed. Despite these limitations, for patients with few treatment options, such as those with PC from appendiceal and CRCs, CRS/HIPEC remains a valuable therapy that can prolong survival and quality of life.