Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Application of biomaterials is one of the most innovative treatments for heart regeneration, involving the use of hydrogels from decellularized extracellular matrix, and their association with nanomaterials, such as alginate, chitosan, hyaluronic acid and gelatin. A promising material is bacterial cellulose hydrogel, due to its nanostructure and morphology being similar to collagen. Cellulose provides support and immobilization of cells, which can result in better cell adhesion, growth and proliferation, making it a safe and innovative material for cardiovascular repair.
- extracellular matrix
- biomaterials
- hydrogels
- bacterial cellulose
1. Bacteria Cellulose: An Innovative Biomaterial
Bacterial cellulose was discovered two centuries ago, however, only in the last few decades, with the development of green chemistry and nanotechnologies, is it gaining space in the research community both in the academic and industrial fields. It is a versatile nanomaterial of commercial interest due to its natural purity, biodegradability, biocompatibility and non-cytotoxicity [1]. Since its discovery, significant research has focused on its production, manufacturing and new applications. Currently, bacterial cellulose has been widely used in drug delivery, tissue engineering, wound dressing, food and cosmetics [2][3].
Cellulose is the most abundant biopolymer on the planet, despite the vast biochemical and phylogenetic diversity of living beings. It is a fibrous, resistant substance insoluble in water, found in the protective cell walls of plants, mainly in stems, trunks and woody portions, being the main structural component of plants giving them mechanical and structural integrity. However, some animals (such as urochordates), fungi and some bacteria also produce it [4][5][6][7][8]. There are other routes of cellulose synthesis, such as chemosynthesis and enzymatic synthesis from glucose derivatives. Cellulose can be classified into two types according to the production origin. There is cellulose from plant biomass, which stands out as a source of raw material for the production of bio-based fuels, paper, packaging and biomedical applications [9][10]. Cellulose is derived from a variety of microorganisms such as fungi, algae (Valonia ventricosae, Glaucocystis), and bacterial strains belonging to the genera Agrobacterium, Aerobacter, Achromobacter, Sarcina, Acetobacter, Rhizobium, Salmonella and Azotobacter that produce acetic acid [11][12][13]. Gluconacetobacter xylinus (formerly known as Acetobacter xylinus and later Komagataibacter xylinus) can produce bacterial cellulose in greater amounts when compared to other species [14]. Such bacteria produce bacterial cellulose in a biosynthetic pathway, involving the secretion of polysaccharides formed while using carbon sources in the medium. Carbon sources such as glucose, sucrose, fructose and glycerin are often used in culture media to produce bacterial cellulose [15]. (Figure 1).
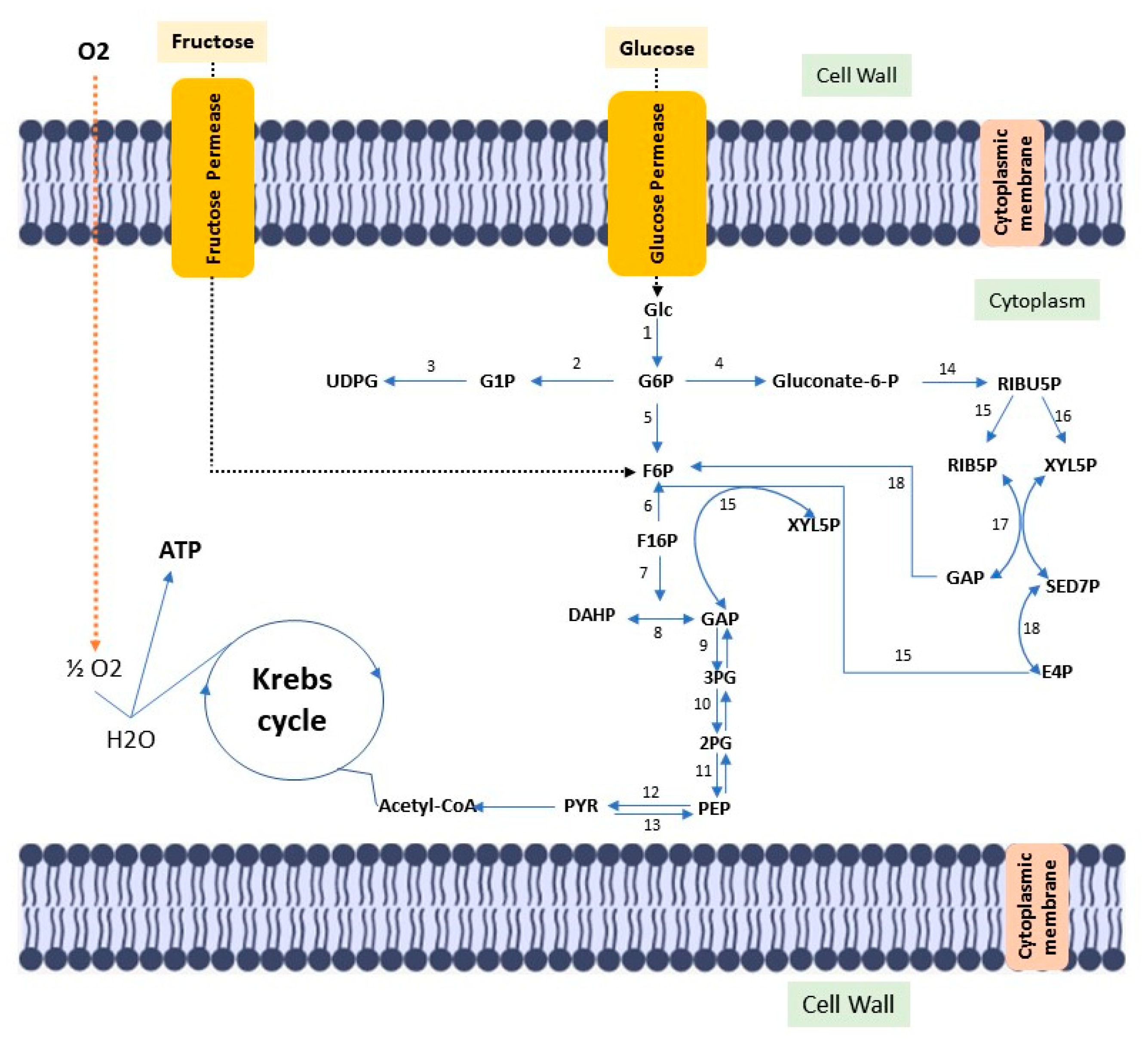
Figure 1. Diagram of the metabolic pathway, stimulated by fructose and glucose, for bacterial cellulose biosynthesis. Glc: Glucose; ATP glucokinase (1); GP6: Glucose 6-phosphate; Phosphoglucomutase (2); G1P: Glucose 1-phosphate; UTP–glucose-1-phosphate uridylyltransferase (3); UDGP: UDP-glucose; Glucose 6-phosphate dehydrogenase (4); Gluconate-6-p: Gluconate-6-phosphate; Phosphoglycoisomerase (5); F6P: fructose 6-phosphate; Fructokinase ATP (6); F16P: Fructose 1,6-bisphosphate; Aldolase (7); Triose phosphate isomerase (8); DHAP: Dihydroxyacetone phosphate; GAP: glyceraldehyde 3-phosphate; Glyceraldehyde 3-phosphate dehydrogenase (9); 3PG: 3-Phosphoglyceric acid; Phosphoglyceratomutase (10); 2PG: 2-Phosphoglyceric acid; Enolase (11); PEP: 2-phosphoenolpyruvate; Pyruvatokinase (12); Pyruvate diphosphate dikinase (13); PYR: Pyruvate; 6-phosphogluconate dehydrogenase (14); RIBU5P: Ribulose 5-phosphate; Phosphorribulose epimerase (15); Phosphorribulose isomerase (16); RIB5P: Ribose 5-phosphate; XYL5P: Xylulose 5-phosphate; Transacetolase (17); SED7P: sedoheptulose 7-phosphate; E4P: Erythrose 4-phosphate; GAP: glyceraldehyde 3-phosphate; Transaldolase (18).
The chemical structure of vegetable cellulose and bacterial cellulose are the same. However, bacterial cellulose has advantages such as high purity (as it is free of lignin and hemicellulose), high crystallinity (84–89%), high water retention (100 times its dry weight), good mechanical properties and 3D nanofibrous structure [16][17][18][19][20] These characteristics make BC a potential material for different applications [21].
Louis Pasteur initially defined bacterial cellulose as a moist, gelatinous skin-like substance produced by the fermentation of coconut water. Later, in 1886, Adrian Brown systematically reported bacterial cellulose as a gelatinous membrane formed on the surface of Bacterium aceti culture medium during acetic acid fermentation [22]. Soon after its discovery, such a membrane was called the “vinegar plant”; the producing microorganism was initially called A. xylinum [23], according to the International Code of Nomenclature of Bacteria, after G. xylinus [24][25]. This genus, Komogataeibacter, is currently the most studied species. It is a strictly aerobic gram-negative bacterium, present in fruits and vegetables in the process of decomposition. It is physiologically characterized by the production of acetic acid from ethanol, by the oxidation of acetate and lactate in carbon dioxide and water, being able to convert common carbon sources (glucose, glycerol, sucrose, fructose mannitol) at temperatures between 25 °C and 30 °C at a pH of 3–7.
There are different fermentation methods and such methods produce bacterial cellulose with different characteristics and applications; there are several methods to control fermentation and increase yield or obtain bacterial cellulose with different characteristics. Commonly, cellulose can be produced by static and agitated cultivation methods. To produce bacterial cellulose, bacteria use several carbon sources, such as glucose, fructose, mannose, glycerin, ethanol and pyruvate [26].
Under static conditions, bacteria need to float on the surface of the medium to obtain oxygen at the surface. Such bacteria produce cellulose at the interface of air and culture medium, similar to a film for a flotation mechanism, which allows the bacteria to stay in the air/liquid near the interface to get the oxygen needed for their metabolism. The film obtained forms a physical barrier protection against UV radiation, increases the ability to colonize other substrates and maintains its hygroscopic nature, enables moisture retention and prevents dehydration [15][21]. Bacterial cellulose produced under static conditions normally has high crystallinity and tensile strength [21]. Under agitation conditions, the bacteria form a fluffy, spherical or irregular cellulose in the medium; Unlike the static method, cultivation under agitation fills the culture medium with oxygen and enables faster cell growth. Cellulose obtained by stirring has a higher water retention capacity, lower Young’s modulus and crystallinity [27]. (Figure 2).
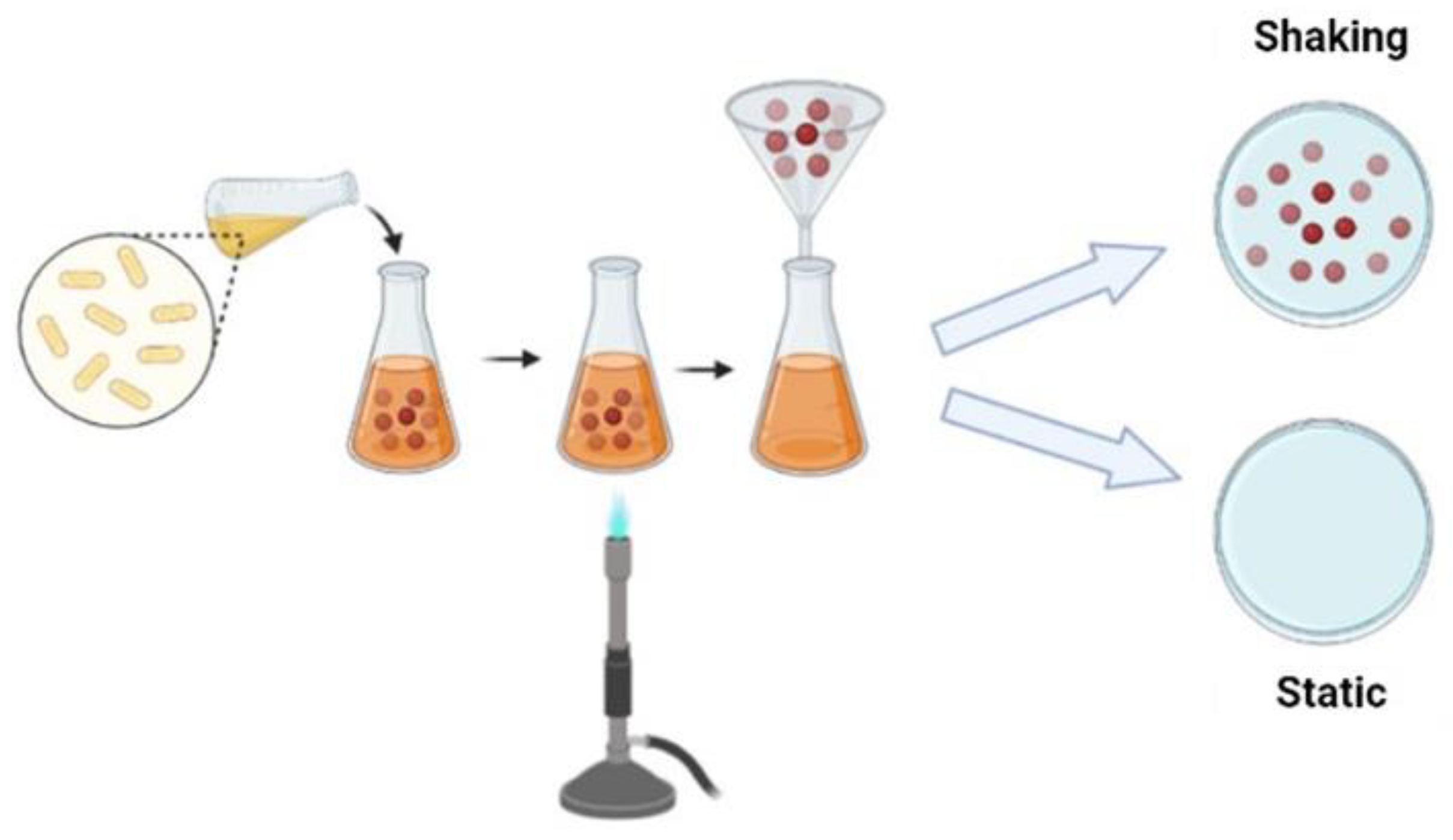
Figure 2. Schematic representation of the BC production strategy.
2. Cellulose Structure
Vegetable cellulose is formed as a lignocellulosic polymer, that is, its cellulose molecules are strongly linked to others, such as lignin and hemicellulose and several others. Any accessory molecules that follow cellulose have specific functionalities in plant physiology. Furthermore, the cellulose content in plants depends on natural sources. Cellulose has a high content of impurities, which requires several molecular adjustments for later application, such as in biomedicine. Furthermore, the purification and isolation of plant cellulose is an arduous process, which involves complex mechanical treatments followed by chemical or enzymatic pre-treatments [28]. The pulp purification processes on an industrial scale generate high costs and great environmental risks due to the degree of toxicity. On the other hand, bacterial cellulose is obtained in a highly pure form, and its purification process is simple, ecologically correct and low cost [29].
Bacterial cellulose is a biomaterial, which can be obtained in a pure form, consisting of glucose and water units. It has a 6-membered cyclic structure with reactive primary and secondary hydroxyl groups; wherein the β-D-glucopyranose ring, all -OH groups are free, playing an essential role for the intermolecular H bond between two adjacent chains. Unlike plant cellulose, bacterial cellulose has a completely crystalline core surrounded by a less crystalline zone interpolated by the amorphous form of cellulose, as well as an arrangement of fibers in a 3D lattice structure. Its fibers tend to self-assemble because of strong interactions and hydroxyl groups, such fibers constitute a network structure interconnected by intramolecular hydrogen bonds, forming sheets with high surface area and high porosity [22]. It contains no hemicellulose or lignin and only a small amount of carboline and carboxyl moieties [30]. The tensile strength of cellulose is between 200–300 MPa, and its Young’s modulus is up to 15–35 GPa [31]. Such mechanical properties are a direct consequence of the crystalline structures of nano and microfibrils. Furthermore, the association of high crystallinity, high content and water are responsible for the thermal stability of the biomaterial [32].
3. Bacteria Cellulose Properties
Bacterial cellulose has several properties, including porosity, mechanical properties, biocompatibility and biodegradability. Some studies in tissue engineering demonstrate that microporous and nanoporous scaffolds are suitable for cell growth [33]. In this way, the 3D porous structure, which allows better cell mobility, is a property of great importance in a biomaterial within tissue engineering, as this characteristic allows better mobility of cells or active agents in the transplant. Bacterial cellulose has membrane pores ranging from 100 to 300 nm, and the lack of macropores restricts the use of cellulose in some biomedical applications. Therefore, the association with gelatin, salt, sugar [34], polyethylene glycol [35], hydroxyapatite [36], sodium with calcium ions [37] is common to increase the porosity of the biomaterial.
The mechanical properties superior to those of vegetable cellulose are attributed to the cross-linked ultrafine fiber structure of bacterial cellulose [38]. Studies have shown that the force-deflection curves in single filaments present a value of 78 ± 17 GPa, as well as fibers aligned with macrofibers based on bacterial cellulose, presented a Young’s modulus of 16.4 GPa and the tensile strength of 248.6 MPa [39][40]. Wang et al. [41] prepared macrofibers based on bacterial cellulose through the drawing and wet twisting process. Such macrofibers showed deformation-dependent mechanical properties, that is, increasing the wet stretching stress, the tensile strength was increased to 826 MPa and the Young’s modulus was 65.7 GPa. Such mechanical properties can be improved with the association of nanomaterials, such as graphene [42][43], graphene oxide [44], silver nanowires [45]; for example. The incorporation of 8% graphene increased tensile strength by 68.8%, while incorporation with 5% graphene oxide improved the Young’s modulus of bacterial cellulose films by 10%, and the 30% graphene increased the tensile strength from ~15 MPa to ~185 MPa.
Biocompatibility can be defined as an adequate host response to the new material in each specific application and the absence of any toxic or allergic effects. Tissue compatibility is a basic and essential prerequisite for a new biomaterial. Such a property is possible due to the 3D nanofibrous network structure that allows cell penetration and proliferation [46]. Bacterial cellulose enables the growth of connective tissue cells, and it is a suitable material for the proliferation of different types of cells [47].
A material must be degraded in a timeframe that responds to the regeneration or healing process. There needs to be an adequate shelf life, no toxicity, and its mechanical properties must be biocompatible with the healing or regeneration process during degradation [34]. It is known that the cellulase enzyme degrades cellulose, and the absence of this enzyme in the human body makes the biomaterial non-biodegradable [48]. In this way, several works seek to increase its degradability, such as associating y-radiations, which degrade rapidly “in vivo” within 2 to 4 weeks [49].
4. Hydrogel: Decellularized Extracellular Matrix and Cellulose
The characteristics of a suitable bioactive hydrogel scaffold need to be similar to the structure and biological properties of the extracellular matrix of natural tissue. Current bioactive polymer hydrogels are limited in simulating various biological functions and mechanical properties of the matrix. A decellularized matrix consists of a natural scaffold prepared from tissues by removing cellular components and retaining the 3D structure of tissues or organs and some components of natural fibers, such as collagen. The scaffold is biocompatible, non-immunogenic and biologically active. A hydrogel-based on the use of decellularized extracellular matrix retains several transforming growth factors, which can enhance cell growth, migration, proliferation, differentiation and angiogenesis; such interaction with cells enables the remodeling of tissue and organ structure and is crucial for the regeneration and functional repair of tissues and organs [50].
Hydrogels from decellularized extracellular matrix have several advantages, such as injectability, since the viscous fluid pre-gel can be injected and polymerized at physiological temperature to form a hydrogel that adapts to the shape of the defect site; having biological activity inherent to the natural matrix; not containing immunogenic cellular material; demonstrate adjustability of their mechanical properties, which can be controlled by concentration or crosslinking. The gelled decellularized matrix has a three-dimensional structure suitable for cell growth. In turn, hydrogels are modifiable and can support cells, therapeutics, drugs and other bioactive molecules. The machinability of hydrogels represented by 3D geometric molecular shapes can be characterized by 3D printing. Thus, the applicability of hydrogels encompasses both “in vivo” tests (in organs such as the heart, liver, lung, brain, colon, spinal cord) and “in vitro” tests (as a substrate for cell culture, biliary tree reconstruction, organoid culture, bioinks derived from the decellularized extracellular matrix) [50][51][52][53] (Figure 3).
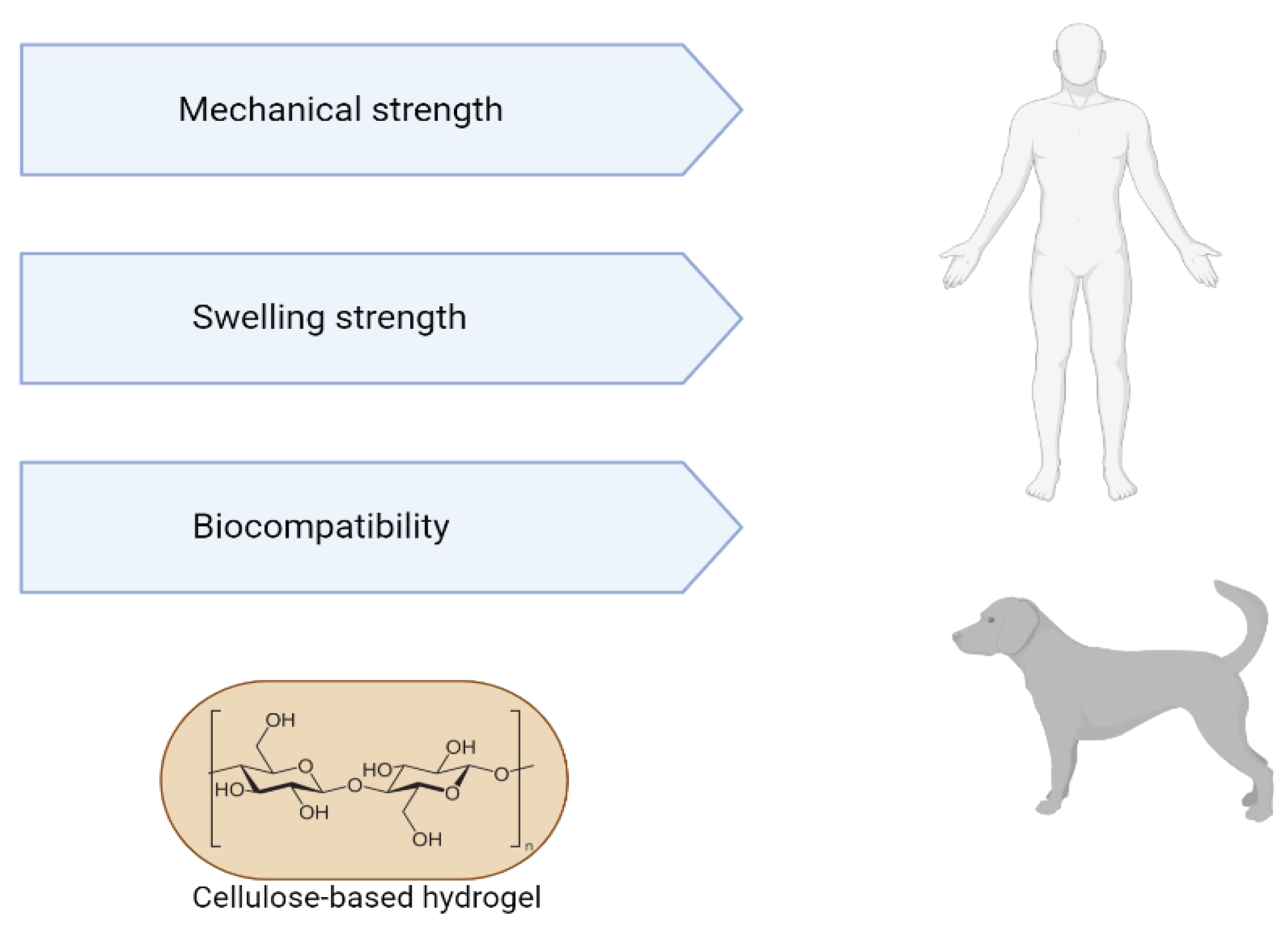
Figure 3. Advantages of using cellulose-based hydrogels for tissue engineering.
The characteristics of scaffolds derived from decellularized extracellular matrix have gained attention in tissue engineering. In the table below, researchers list some studies that produced extracellular matrix hydrogels for cardiac tissue regeneration.
Cellulose-based hydrogels are used in various fields related to tissue engineering, such as bioactive cartilage implants; prototypes of blood vessels [54]; dressings [55]; surgical implants [56]; drug delivery [57]; artificial corneal grafts [58]; and dental implants [59]. Some BC-based products have already been commercialized, such as BioFill®, Bioprocess®, XCell® and DermafillTM, which are examples of bio-based membranes that have the main characteristics necessary for an ideal dressing [57]. BASYC® is used for artificial blood vessels and Gegiflex® is available for tissue engineering [59]. Bacterial nanocellulose (NCB) has enormous potential for use as a scaffold in tissue engineering, as bacterial cellulose is more effective than plant cellulose, which justifies the fact that bacterial cellulose is the first choice in medical and health applications for tissue engineering [58]. This biomaterial has promising characteristics due to the similarity of its nanostructure and morphology to collagen, which makes cellulose an option for use in supporting and immobilizing cells. The architecture of bacterial cellulose-based materials can be designed at different scales, from the nano to the macroscale, controlling the biomanufacturing process. BC fibers are solid and, when used in combination with other biocompatible materials, produce nanocomposites particularly suitable for use in human and veterinary medicine [60].
Although bacterial cellulose has several properties that are of great value for tissue engineering and for several biomedical applications, numerous approaches are applied to change its physical–chemical and functional properties, such as porosity, crystallinity, chemical structures and functions, to fully explore the potential of bacterial cellulose. Bacterial cellulose can undergo both in situ and ex situ modifications (Figure 3). The in situ modification describes the exogenous molecules addition to the culture medium during cellulose biosynthesis, while the ex-situ modification describes the materials inclusion after bacterial cellulose biosynthesis and purification [15]. Such approaches seek to modify bacterial cellulose in order to expand its advantageous characteristics and solve its disadvantages (Figure 4).

Figure 4. Advantages and disadvantages of bacterial cellulose and solutions to improve its properties.
The new in situ properties interfere with the nanofibers crosslinking. The main objective of such modification are new characteristics in the matrix, changing its biophysical properties. The additives become part of the nanofibers, interacting with the –OH portions present in the bacterial cellulose chains and forming new hydrogen bonds. Chitosan, a polysaccharide derived from chitin, has biocompatibility, antibacterial and antifungal properties. The combination of bacterial cellulose, in a dressing, exhibited favorable antibacterial activities and no cytotoxicity [61]. Zhou et al. [62] demonstrated that their bacterial cellulose bandage associated with collagen I and hydroxypropyltrimethyl ammonium chloride chitosan exhibited excellent antibacterial activity, cytocompatibility and promoted the growth and proliferation of NIH3T3 cells and HUVECs cells. Silver nanoparticles and polydopamine incorporated into bacterial cellulose demonstrated antibacterial activity, increased cell viability, showed no cytotoxicity to fibroblast cells, granulation tissue formation, angiogenesis and re-epithelialization upon histopathological examination [63]. Several nanotubes, nanosheets were also incorporated into cellulose culture media. Park et al. [64] produced hybrid compounds of bacterial cellulose and carbon nanotubes that showed osteoconductivity and osteoinductivity. Likewise, Khalid et al. [65] demonstrated that the bandage, composed of bacterial cellulose and carbon nanotubes, acted as a mechanical and antibacterial barrier to fragile healing tissue, aided in moisture retention, reduced inflammation, and resulted in efficient wound healing. Graphene nanosheets were incorporated into the bacterial cellulose matrix, resulting in decreased crystallinity, improved mechanical and electrical properties. Luo et al. [66] produced a compound that exhibited high tensile strength with 93% improvement compared to pure bacterial cellulose film. In addition, the film also showed excellent flexibility with good conductivity. The association between nano zinc oxide and bacterial cellulose increased porosity and pore sizes, which increased water vapor permeability (an important factor for a bandage), it also showed antibacterial activity, good physical properties, non-cytotoxicity and good biocompatibility [67]. Although in situ modifications allow a uniform material distribution, the fermentation conditions of the biosynthesis process limit the incorporation of other materials.
Ex-situ modifications seek to alter the physicochemical and functional properties of the matrix after biosynthesis and purification of bacterial cellulose. The nanometric materials can be aggregated through diffusion to pass through the network pores. This type of modification can be divided into the chemical modification and composites development [68].
In the chemical modification process, bacterial cellulose is treated with several chemical reagents to modify its chemical structure and incorporate additional functionalities. The most common chemical modification is oxidation but there are also modifications by acetylation [69], benzoylation [70], succinylation [71] and phosphorylation [72].
Oxidation seeks to add new functional groups to cellulose. Oxidized cellulose is the most precious by-product of cellulose, and several chemical and physical properties of oxidized cellulose can be obtained under various oxidizing conditions (nature, temperature, pH and reaction duration) [73]. Many agents can be used, such as hydrogen peroxide, persulfates, permanganates, nitrogen dioxide, chlorine dioxide and phosphoric acids [74]. However, water-soluble 2, 2, 6, 6-tetramethylpiperidine-1-oxyl (TEMPO) is widely used to oxidize cellulose. Oxidized BC has been investigated for different applications such as adsorption of heavy metals, oil removal and various biomedical applications [75][76]. In etherification, the reaction is carried out in two steps; in the first step, cellulose is activated by treatment with an alkaline solution, followed by an etherification reaction with monochloroacetic acid or its sodium salt. Carboxymethylcellulose is one of the most important cellulose derivatives and can be used in pharmaceutical, cosmetic, food and biomedical areas [77]. Sulfation synthesizes cellulose in sulfuric acid in isopropyl alcohol or with SO3-pyridine complex in ionic liquids [78][79]. Cellulose sulfate has as its main characteristic, anticoagulating, antivirus and antibacterial properties [80]. Benzoylation treats bacterial cellulose with benzoyl citrate, adding to the material the potential for sensors, piezoelectric materials and optical properties [81]. Phosphorylation is developed for textiles and flame retardant materials, as it can induce the formation of calcium phosphate making the material suitable for biomedical applications [16].
Despite its advantages, cellulose has no antibacterial capacity and moderate mechanical properties. The development of composites aims to improve some properties that limit the application of bacterial cellulose in biomedical and tissue engineering. To improve mechanical and biological properties, researchers have incorporated different types of materials into bacterial cellulose, including polymers, carbon-based nanoparticles, metal/metal oxide nanoparticles, and other inorganic nanoparticles [82].
Bacterial cellulose fragments were immersed in the chitosan solution followed by lyophilization to produce a scaffold to aid in ovarian cancer diagnosis. The scaffold obtained showed better interaction with the cells compared to pure BC [83]. JU et al. [84] produced a bacterial cellulose film, in which the cellulose suspension and the polyvinyl alcohol solution were mixed, followed by the incorporation of chitosan in bulk form or nanoparticle form. The bulk form of chitosan increased the mechanical and elastic properties of the film, while the nanoparticle form showed higher antibacterial properties. The gelatin and hydroxyapatite incorporation in bacterial cellulose showed a composite with high mechanical properties, positive cell adhesion, proliferation and differentiation [85]. Yan et al. [86] achieved a scaffold with reduced porosity, high mechanical properties and great in vitro biocompatibility, by incorporating bacterial nano-cellulose into alginate and collagen. The incorporation of graphene, a carbon nanomaterial with a 2D structure, and carbon nanotubes add to bacterial cellulose better mechanical, electrical and thermal properties [16][87].
In summary, several biopolymers and biomaterials can be incorporated into bacterial cellulose to improve its properties, reducing its applicability limitations. The in situ and ex-situ modifications are methods that work on the incorporation of these materials homogeneously. Although the in situ modifications present several advantages of materials aggregation, the method is limited because some materials do not support the biosynthetic process. On the other hand, ex-situ modification expands the range of materials that can be incorporated; however, scientists still seek completely homogeneous incorporation in this process.
5. Bacterial Cellulose for Cardiac Tissue Regeneration
Understanding the environment nanoscale is essential to produce biomaterials that mimic the cellular microenvironment. The environment properties employ a total influence on cell adhesion, proliferation, maturation and differentiation, and consequently generate impacts on the function of a tissue. Cellulose is a very versatile material with its adaptable properties that allow its application in systems with different chemical and biophysical environments. Cellulose-based biomaterials provide important advantages over conventional synthetic materials, which demonstrates their promise of advancing scientific knowledge. The role of the extracellular matrix is established, and researchers know that it not only allows cellular attachment but also sends biochemical and biophysical clues to the nascent cells and tissues. Such data support studies on the application of scaffolds of decellularized tissues and organs in tissue engineering and regenerative medicine. The mimicry of natural conditions both in the tissue and in the ECM requires adequate adhesion and growth properties that maintain the tissue’s normal structure, and the results of biopolymers’ application involving celluloses mentioned above reveal successful results.
The bacterial cellulose use in cardiac tissue regeneration still needs more studies. In the literature, only one study was found that tested the cellulose membrane viability, acting as an adhesive, loaded with co-cultured cells. Simeoni et al. [88] produced a patch loaded with skeletal myoblasts and mesenchymal stem cells that was surgically inserted into the epicardial region of the left ventricle, where they found that the cellulose patch can protect the myocardium against the deleterious effects and pathological remodeling of the ischemic heart; this beneficial result was not obtained only with cell therapy. Other studies demonstrated the applicability of cellulose, modified cellulose and its composites. Only Simoeni et al. [88] describe the bacterial cellulose use itself. Chen et al. [89] developed a polyurethane/cellulose scaffold that presented greater mechanical strength and essential characteristics for the survival and function of cardiac cells with native anisotropy. As such, Entcheva et al. [90] tested the potential of cellulose acetate and reduced cellulose scaffolds for the growth of cardiomyocytes in vitro. They attested that the surface of these materials promoted cell growth, while increasing gap junctions, and electrical functionality. Such studies open doors to new possibilities for applications of bacterial cellulose, at the same time highlighting the potential of this biomaterial in cardiac tissue regeneration.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23073955
References
- Raghavendran, V.; Asare, E.; Roy, I. Bacterial cellulose: Biosynthesis, production, and applications. Adv. Microb. Physiol. 2020, 77, 89–138.
- de Oliveira Barud, H.G.; Da Silva, R.R.; Barud, H.D.S.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; De Oliveira, O.B., Jr.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial celulose. Carbohydr. Polym. 2016, 153, 406–420.
- Blanco Parte, F.G.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current progress on the production, modification, and applications of bacterial celulose. Crit. Rev. Biotechnol. 2020, 40, 397–414.
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207.
- Schurz, J. “Trends in polymer science”. A bright future for cellulose. Prog. Polym. Sci. 1999, 24, 481–483.
- Eichhorn, S.J.; Young, R.J.; Davies, G.R. Modeling Crystal and Molecular Deformation in Regenerated Cellulose Fibers. Biomacromolecules 2005, 6, 507–513.
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352.
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: A review ACS Sustain. Chem. Eng. 2018, 6, 2807–2828.
- Lee, S.Y.; Kim, H.U.; Chae, T.U.; Cho, J.S.; Kim, J.W.; Shin, J.H.; Kim, D.I.; Ko, Y.S.; Jang, W.D.; Jang, Y.S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019, 2, 18–33.
- Rizal, S.; Khalil, A.H.P.S.; Oyekanmi, A.A.; Gideon, O.N.; Abdullah, C.K.; Yahya, E.B.; Alfatah, T.; Sabaruddin, F.A.; Rahman, A.A. Cotton Wastes Functionalized Biomaterials from Micro to Nano: A Cleaner Approach for a Sustainable Environmental Application. Polymers 2021, 13, 1006.
- Reiniati, I.; Hrymak, E.; Margaritis, A. Desenvolvimentos recentes na produção e aplicações de fibras de celulose bacteriana e nanocristais. Crit. Rev. Biotechnol. 2017, 37, 510–524.
- Lin, D.H.; Liu, Z.; Shen, R.; Chen, S.Q.; Yang, X.B. Bacterial cellulose in food industry: Current research and future prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019.
- Maric, L.; Cleenwerck, I.; Accetto, T.; Vandamme, P.; Trcek, J. Description of Komagataeibacter melaceti sp. nov. and Komagataeibacter melomenusus sp. nov. Isolated from Apple Cider Vinegar. Microorganisms 2020, 8, 1178.
- Lu, T.F.; Gao, H.L.; Liao, B.W.; Wu, J.J.; Zhang, W.; Huang, J.; Liu, M.Y.; Huang, J.; Chang, Z.Y.; Jin, M.F.; et al. Characterization and optimization of production of bacterial cellulose from strain CGMCC 17276 based on whole-genome analysis. Carbohydr. Polym. 2020, 232, 115788.
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in bacterial cellulose matrices for biotechnological applications. Bioresour. Technol. 2016, 213, 172–180.
- Higuchi, A.; Aoki, N.; Yamamoto, T.; Miyazaki, T.; Fukushima, H.; Tak, T.M.; Jyujyoji, S.; Egashira, S.; Matsuoka, Y.; Natori, S.H. Temperature-induced cell detachment on immobilized pluronic surface. J. Biomed. Mater. Res. A 2006, 79, 380–392.
- Barud, H.S.; Regiani, T.; Marques, R.F.C.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J.L. Antimicrobial bacterial cellulose-silver nanoparticles composite membranes. J. Nanomater. 2011, 2011, 721631.
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Bacterial cellulose/poly (3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284.
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603.
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mat. Sci. Eng. C Mater. 2018, 82, 372–383.
- Wahid, F.; Huang, L.-H.; Zhao, X.-Q.; Li, W.-C.; Wang, Y.-Y.; Jia, S.-R.; Zhong, C. Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 2021, 53, 107856.
- Foresti, M.L.; Vázquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467.
- Lin, S.-P.; ILoira Calvar Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial celulose. Cellulose 2013, 20, 2191–2219.
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bactéria. Microbiol. Rev. 1991, 55, 35–58.
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and properties of nanofibrous bacterial cellulose-containing polymer composites: A review of recent advances for biomedical applications. Polym. Rev. 2020, 60, 144–170.
- Matsuoka, M.; Tsuchida, T.; Matsushita, K.; Adachi, O.; Yoshinaga, F. A synthetic medium for bacterial cellulose production by acetobacter xylinum subsp. Sucrofermentans. Biosci. Biotechnol. Biochem. 1996, 60, 575–579.
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 1998, 5, 187–200.
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494.
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545.
- Schubert, S.; Schlufter, K.; Heinze, T. Configurations, structures, and morphologies of cellulose. In Polysaccharides in Medicinal and Pharmaceutical Applications; Popa, V., Ed.; iSmithers: Shrewsbury, UK, 2011; pp. 1–55.
- Ruka, D.R.; Simon, G.P.; Dean, K.M. Bacterial cellulose and its use in renewable composites. In Nanocellulose Polymer Nanocomposites: Fundamentals and Applications; Thakur, V.J., Ed.; Scrivener Publishing LLC: Salem, MA, USA, 2014; pp. 89–130.
- Qiu, K.; Netravali, A.N. A Review of fabrication and applications of bacterial cellulose based nanocomposites. Polym. Rev. 2014, 54, 598–626.
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.F.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141.
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. 2018, 11, 34–49.
- Dubey, S.; Mishra, R.; Roy, P.; Singh, R.P. 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with bmp-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 2020, 167, 934–946.
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.N.; Zhang, X.L.; Dai, K.R. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mat. Sci. Eng. C Mater. 2017, 75, 1034–1041.
- Kirdponpattara, S.; Khamkeaw, A.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Structural modification and characterization of bacterial cellulose-alginate composite scaffolds for tissue engineering. Carbohydr. Polym. 2015, 132, 146–155.
- Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S.; Nishi, Y.; Uryu, M. The structure and mechanical properties of sheets prepared from bacterial celulose. J. Mater. Sci. 1989, 24, 3141–3145.
- Guhados, G.; Wan, W.K.; Hutter, J.L. Measurement of the elastic modulus of single bacterial cellulose fibers using atomic force microscopy. Langmuir 2005, 21, 6642–6646.
- Yao, J.J.; Chen, S.Y.; Chen, Y.; Wang, B.X.; Pei, Q.B.; Wang, H.P. Macrofibers with high mechanical performance based on aligned bacterial cellulose nanofibers. ACS Appl. Mater. Interfaces 2017, 9, 20330–20339.
- Wang, S.; Jiang, F.; Xu, X.; Kuang, Y.; Fu, K.; Hitz, E.; Hu, L. Super-strong, super-stiff macrofibers with aligned, long bacterial cellulose nanofibers. Adv. Mater. 2017, 29.
- Shao, W.; Wang, S.; Liu, H.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Preparation of bacterial cellulose/graphene nanosheets composite films with enhanced mechanical performances. Carbohydr. Polym. 2016, 138, 166–171.
- Luo, H.; Dong, J.; Xu, X.; Wang, J.; Yang, Z.; Wan, Y. Exploring excellent dispersion of graphene nanosheets in three-dimensional bacterial cellulose for ultra-strong nanocomposite hydrogels. Compos. Part A Appl. Sci. 2018, 109, 290–297.
- Ccorahua, R.; Troncoso, O.P.; Rodriguez, S.; Lopez, D.; Torres, F.G. Hydrazine treatment improves conductivity of bacterial cellulose/graphene nanocomposites obtained by a novel processing method. Carbohydr. Polym. 2017, 171, 68–76.
- Wan, Y.; Yang, S.; Wang, J.; Gan, D.; Gama, M.; Yang, Z.; Zhu, Y.; Yao, F.; Luo, H. Scalable synthesis of robust and stretchable composite wound dressings by dispersing silver nanowires in continuous bacterial celulose. Compos. Part B Eng. 2020, 199, 1082.
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151.
- Zhang, J.; Chang, P.; Zhang, C.; Xiong, G.; Luo, H.; Zhu, Y.; Ren, K.; Yao, F.; Wan, Y. Immobilization of lecithin on bacterial cellulose nanofibers for improved biological functions. React. Funct. Polym. 2015, 91–92, 100–107.
- Torgbo, S.; Sukyai, P. Biodegradation and thermal stability of bacterial cellulose as biomaterial: The relevance in biomedical applications. Polym. Degrad. Stab. 2020, 179, 109232.
- Czaja, W.; Kyryliouk, D.; Depaula, C.A.; Buechter, D.D. Oxidation of gamma-irradiated microbial cellulose results in bioresorbable, highly conformable biomaterial. J. Appl. Polím. Sci. 2014, 131, 1018.
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen. Ther. 2021, 18, 88–96.
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038.
- Ungerleider, J.L.; Johnson, T.D.; Rao, N.; Christman, K.L. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods 2015, 84, 53–59.
- Lin, T.; Liu, S.; Chen, S.; Qiu, S.; Rao, Z.; Liu, J.; Zhu, S.; Yan, L.; Mao, H.; Zhu, Q.; et al. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects. Acta Biomater. 2018, 73, 326–338.
- Avery, N.C.; Sims, T.J.; Warkup, C.; Bailey, A.J. Collagen cross-linking in porcine m. longissimus lumborum: Absence of a relationship with variation in texture at pork weight. Meat Sci. 1996, 42, 355–369.
- Shah, J.; Malcolm, B.R. Towards electronic paper displays made from microbial cellulose. Appl. Microbiol. Biotechnol. 2004, 66, 352–355.
- Millon, L.E.; Wan, W.K. The polyvinyl alcohol-bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 79, 245–253.
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12.
- Dutta, S.D.; Patel, D.K.; Lim, K.T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55.
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571.
- Mohite, B.V.; Koli, S.H.; Patil, S.V. Bacterial cellulose-based hydrogels: Synthesis, Properties, and Applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, I.H., Ed.; Springer Nature: Cham, Switzerland, 2018; pp. 1–22.
- Ao, H.; Jiang, W.; Nie, Y.; Zhou, C.; Zong, J.; Liu, M.; Liu, X.; Wan, Y. Engineering quaternized chitosan in the 3D bacterial cellulose structure for antibacterial wound dressings. Polym. Test. 2020, 86, 106490.
- Zhou, C.; Yang, Z.; Xun, X.; Ma, L.; Chen, Z.; Hu, X.; Wu, X.; Wan Ao, H. De novo strategy with engineering a multifunctional bacterial cellulose-based dressing for rapid healing of infected wounds. Bioact. Mater. 2022, 13, 212–222.
- Jiji, S.; Udhayakumar, S.; Maharajan, K.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Bacterial cellulose matrix with in situ impregnation of silver nanoparticles via catecholic redox chemistry for third degree burn wound healing. Carbohydr. Polym. 2020, 245, 116573.
- Park, S.; Park, J.; Jo, I.; Cho, S. In situ hybridization of carbon nanotubes with bacterial cellulose for three-dimensional hybrid bioscaffolds. Biomaterials 2015, 58, 93–102.
- Khalid, A.; Madni, A.; Raza, B.; Islam, M.; Hassan, A.; Ahmad, F.; Ali, H.; Khan, T.; Kahid, F. Multiwalled carbon nanotubes functionalized bacterial cellulose as an efficient healing material for diabetic wounds. Int. J. Biol. Macromol. 2022, 203, 265–267.
- Luo, H.; Xie, J.; Xiong, L.; Zhu, Y.; Yang, Z.; Wan, Y. Fabrication of flexible, ultra-strong, and highly conductive bacterial cellulose-based paper by engineering dispersion of graphene nanosheets. Compos. Part B Eng. 2019, 162, 484–490.
- Luo, Z.; Liu, J.; Lin, H.; Ren, X.; Tian, H.; Liang, Y.; Wang, W.; Wang, Y.; Yin, M.; Huang, Y.; et al. In situ Fabrication of Nano ZnO/BCM Biocomposite Based on MA Modified Bacterial Cellulose Membrane for Antibacterial and Wound Healing. Int. J. Nanomed. 2020, 15, 1–15.
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598.
- Ifuku, S.; Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Yano, H. Surface modification of bacterial cellulose nanofibers for property enhancement of optically transparent composites: Dependence on acetyl-group DS. Biomacromolecules 2007, 8, 1973–1978.
- Wang, Y.; Luo, Q.; Peng, B.; Pei, C. A novel thermotropic liquid crystalline—Benzoylated bacterial celulose. Carbohydr. Polym. 2008, 74, 875–879.
- Yin, X.; Yu, C.; Zhang, X.; Yang, J. Comparison of succinylation methods for bacterial cellulose and adsorption capacities of bacterial cellulose derivatives for Cu 2+ ion. Polym. Bull. 2011, 67, 401–412.
- Oshima, T.; Kondo, K.; Ohto, K.; Inoue, K. Preparation of phosphorylated bacterial cellulose as an adsorbent for metal ions. React. Funct. Polym. 2008, 68, 376–383.
- Zhang, S.; Li, J.; Chen, S.; Zhang, X.; Ma, J.; He, J. Oxidized cellulose-based hemostatic materials. Carbohydr. Polym. 2020, 230, 115585.
- Solomevich, S.O.; Dmitruk, E.I.; Bychkovsky, P.M.; Nebytov, A.E.; Yurkhtovich, T.L.; Golub, N.V. Fabrication of oxidized bacterial cellulose by nitrogen dioxide in chloroform/cyclohexane as a highly loaded drug carrier for sustained release of cisplatin. Carbohydr. Polym. 2020, 248, 116745.
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.; Lee, B. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313.
- Weyell, P.; Beekmann, U.; Kupper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-made material characteristics of bacterial cellulose for drug delivery applications in dentistry. Carbohydr. Polym. 2019, 207, 1–10.
- Casaburi, A.; Rojo, U.M.; Carrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial celulose. Food Hydrocoll. 2018, 75, 147–156.
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Al-Salame, H.; Dykes, G.A.; Coorey, R. Antimicrobial efficacy of nisin-loaded bacterial cellulose nanocrystals against selected meat spoilage lactic acid bacteria. Carbohydr. Polym. 2021, 251, 117096.
- Zhang, K.; Peschel, D.; Baucker, E.; Groth, T.; Fischer, S. Synthesis and characterisation of cellulose sulfates regarding the degrees of substitution, degrees of polymerisation and morphology. Carbohydr. Polym. 2011, 83, 1659–1664.
- Peschel, D.; Zhang, K.; Aggarwal, N.; Brendler, E.; Fischer, S.; Groth, T. Synthesis of novel celluloses derivatives and investigation of their mitogenic activity in the presence and absence of FGF2. Acta Biomater. 2010, 6, 2116–2125.
- Zhou, M.; Duan, X.; Ma, Y.; Zhou, Y. Morphology control and optical properties of organic nanostructures based on thermotropic liquid crystalline benzoylated bacterial celulose. Carbohydr. Polym. 2010, 80, 551–554.
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293.
- Ui-Islam, M.; Subhan, F.; Ui Islam, S.; Khan, S.; Shah, N.; Manan, S.; Ullah, M.W.; Yang, G. Development of three-dimensional bacterial cellulose/chitosan scaffolds: Analysis of cell-scaffold interaction for potential application in the diagnosis of ovarian cancer. Int. J. Biol. Macromol. 2019, 137, 1050–1059.
- Ju, S.; Zhang, F.; Duan, J.; Jiang, J. Characterization of bacterial cellulose composite films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2020, 237, 116167.
- Ran, J.; Jiang, P.; Liu, S.; Sun, G.; Yan, P.; Shen, X.; Tong, H. Constructing multi-component organic/inorganic composite bacterial cellulose-gelatin/hydroxyapatite double-network scaffold platform for stem cell-mediated bone tissue engineering. Mater. Sci. Eng. C 2017, 78, 130–140.
- Yan, H.; Huang, D.; Chen, X.; Liu, H. A novel and homogeneous scaffold material: Preparation and evaluation of alginate/bacterial cellulose nanocrystals/collagen composite hydrogel for tissue engineering. Polym. Bull. 2018, 75, 1–16.
- Dong, Y.; Zhang, H.; Zhong, G.; Yao, G.; Lai, B. Cellulose/carbon Composites and their Applications in Water Treatment—A Review. Chem. Eng. J. 2021, 405, 126980.
- Simeoni, R.B.; Mogharbel, B.F.; Francisco, J.C.; Miyague, N.I.; Irioda, A.C.; Souza, C.M.C.O.; Souza, D.; Stricker, P.E.F.; da Rosa, N.N.; Souza, C.F.; et al. Beneficial Roles of Cellulose Patch-Mediated Cell Therapy in Myocardial Infarction: A Preclinical Study. Cells 2021, 10, 424.
- Chen, P.; Liao, H.C.; Hsu, S.H.; Wu, M.C.; Yang, Y.F.; Wu, C.C.; Chen, M.H.; Su, W.F. A novel polyurethane/cellulose fibrous scaffold for cardiac tissue engineering. RSC Adv. 2015, 5, 6932–6939.
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.Y.; Farrel, M.; Kostov, Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials 2004, 25, 5753–5762.
This entry is offline, you can click here to edit this entry!
