Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Src non-receptor tyrosine kinase phosphorylates a variety of protein substrates that perform specific cellular functions. Activity of Src is regulated by a variety of stimuli and the Src protein is subjected to several types of post-translational modifications including lipidation, phosphorylation, acetylation, ubiquitylation, sumoylation and oxidation. In particular, p-Tyr416 Src has been known to be an active form while p-Tyr527 Src is an inactive form through autoinhibition by binding to Src SH2 own domain.
- GSK-3β
- β-catenin
- Rho GTPases
- Src
- Tyrosine Kinase
- Non-receptor tyrosine kinase
1. Src Non-Receptor Tyrosine Kinase
Src non-receptor tyrosine kinase phosphorylates a variety of protein substrates that perform specific cellular functions. Activity of Src is regulated by a variety of stimuli and the Src protein is subjected to several types of post-translational modifications including lipidation, phosphorylation, acetylation, ubiquitylation, sumoylation and oxidation. In particular, p-Tyr416 Src has been known to be an active form while p-Tyr527 Src is an inactive form through autoinhibition by binding to Src SH2 own domain (Figure 1). Src also plays specific roles in specific compartments of the cell as Src is localized to the membrane, cytosol, mitochondria, and nucleus for specific functions [1]. Here is the regulation of Src activities concurrent with post-translational modification (Table 1).
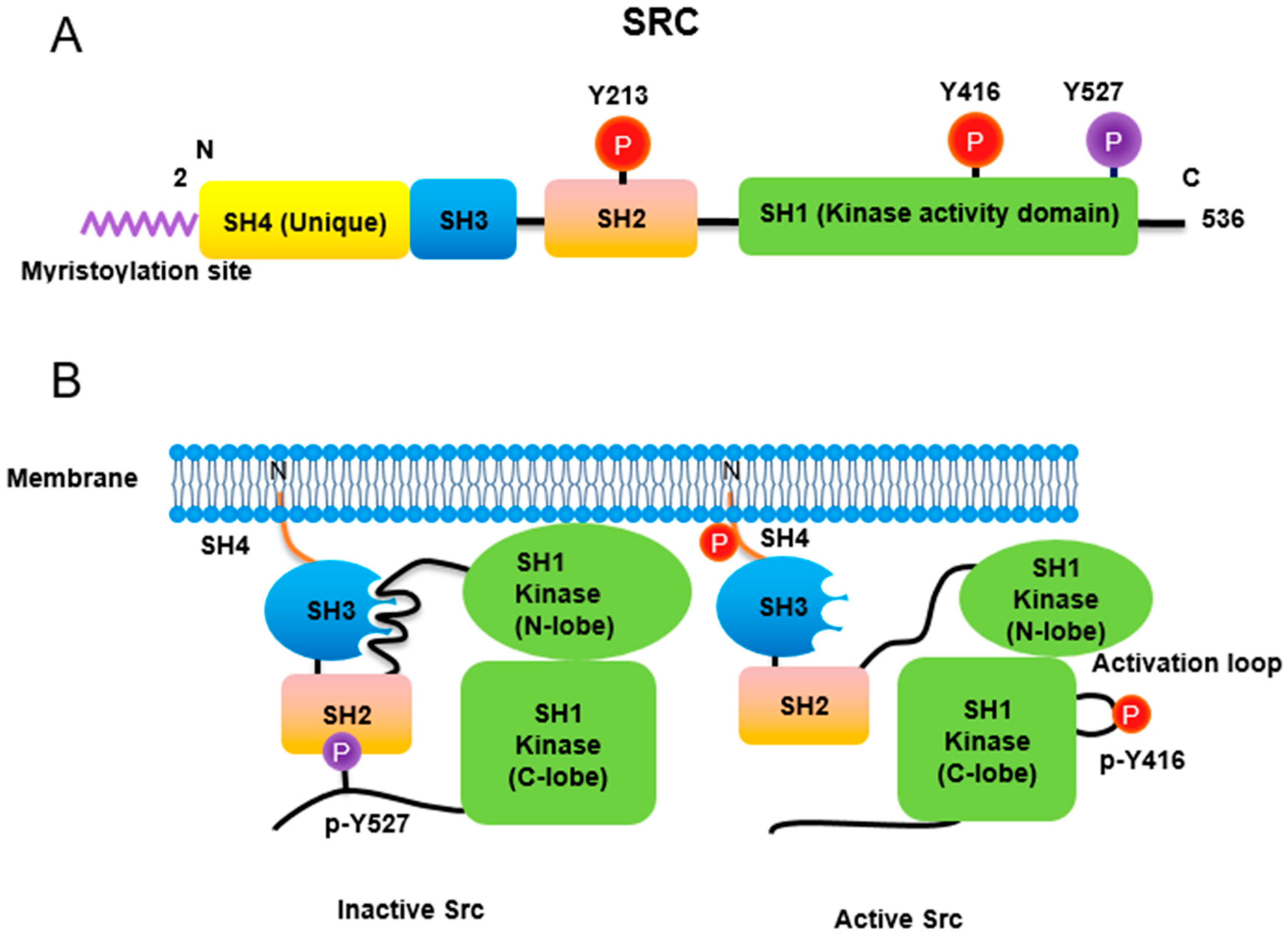
Figure 1. Regulation of Src non-receptor tyrosine kinase activity. (A) Src consists of several domains including SH1, SH2, SH3 and SH4 domains. Important Tyr phosphorylation sites of Y527, Y416 and Y213 are noted. (B) Tyr527 phosphorylated by C-terminal Src kinase (CSK) binds to SH2 domain in Src, leading to the inhibition of Src activity. Phosphorylated Tyr213 of Src interferes with SH2 domain binding to p-Tyr527 residue of Src. Dephosphorylation of Src Tyr527 by receptor protein tyrosine phosphatase α activates Src and Tyr416 phosphorylation acquires a fully activation.
2. Lipidation
Myristic acid is covalently linked to Gly2 instead of the Met initiator amino acid in Src. This myristoylation of Src is required for its membrane association, and cell transformation. Furthermore, the first 14 amino acids of Src contain a recognition sequence for its myristoylation [2][3]. Myristoylation is carried out by N-myristoyl transferase (NMT) by using myristoyl-CoA, with the consensus sequence for NMT protein substrate being Met-Gly-X-X-X-Ser/Thr. The initiating Met is cleaved by methionine amino peptidase and Gly2 becomes the N-terminal amino acid of the myristoylated Src [4].
Myristoylation stimulates Src activity by anchoring it to lipid membranes and the membrane binding also regulates Src ubiquitylation and degradation, as a nonmyristoylated Src has reduced activity, but it has enhanced stability. Src also has a hydrophobic pocket domain in the C-terminal region that binds to the myristoyl group. T456A mutation of Src leads to detachment from the lipid membrane, suggesting that Thr456 is localized to a binding pocket; this mutation also seems to promote to a nonmyristoylation state [5]. The N-terminal myristoylated SH4 domain of Src was recently reported to interact with its SH3 domain when Src is not anchored to a lipid membrane, and the residues in the domain termed the Unique lipid binding domain modulate this interaction [6]. For Src, in addition to its myristoyl group, six basic residues in the amino terminus (Myristoyl-GSSKSKPKDPSQRRR) are particularly essential for high affinity binding to the lipid bilayer via electrostatic interaction with acidic phospholipid, shown in vitro [7].
Other SFKs including Yes, Fyn, Lyn, Lck, Hck, Fgr, and Yrk harbor cysteine residue(s) in their N-terminal domain that can be covalently linked to palmitic acid by palmitoyl acyltransferase (PAT); this enzyme recognizes Met-Gly-Cys at the N-termini of these SFKs. However, Src does not contain this cysteine residue, and thus cannot be modified with palmitoylation [4].
3. Phosphorylation
3.1. Phosphorylation of Tyr416 and Tyr527
Similar to most protein kinases, Src family kinases also require phosphorylation for regulation of their enzyme activity. Autophosphorylation of Src at Tyr416 (chicken; Tyr419 in human) ensures an active form [8]. In contrast, phosphorylation of Src at Tyr527 (chicken; Tyr530 in human) in the Src C-terminal domain inactivates the enzyme [9]. Tyr527 phosphorylation is carried out by C-terminal Src kinase (Csk) [10] and its homolog, Csk homologous kinase (Chk) [11][12]. Phosphorylation of the C-terminal Tyr527 of Src promotes binding of this phosphorylated residue to its own SH2 domain, resulting in an intramolecular auto-inhibitory Src structure [13][14]. Regarding the control of Src activity via Tyr527, receptor protein tyrosine phosphatase α (RPTPα) -/- mouse has impaired Src activity, manifested by a concomitant increase in phosphorylated Tyr527 forms of Src [15][16]. In addition, PDGF receptor, being a membrane tyrosine kinase, is able to phosphorylate Src at its Tyr213 residue near the binding pocket of SH2 domain, which then interferes with p-Tyr527 residue binding to the Src SH2 domain, leading to Src activation [17]. A detailed three-dimensional structure of Src with phosphorylations of Tyr416 and Tyr527 has also been described [18].
3.2. Phosphorylation of Ser17, Ser37, Ser69, and Ser75 in the Unique Domain of Src
Src protein has several domains including SH1 (catalytic), SH2, SH3, SH4, and Unique domains. The Src Unique domain localized in the N-terminal region of the protein exhibits strong sequence divergence from the other SFK members. Recently, the Unique domain was reported to reveal a crucial role in regulation of Src activity. In particular, phosphorylation/dephosphorylation observed in the Unique domain of Src plays a critical regulatory function in Src kinase activity. There is also the unique lipid binding region (ULBR) that is a partially structured region within the Unique domain of Src [19].
Platelet-derived growth factor (PDGF) activation of the cells causes Src to translocate from the plasma membrane to the cytosol and a 4-fold activation of its kinase activity and phosphorylation of its N-terminal region. Ser17 of Src was postulated to be a candidate phosphorylation residue, which likely changes the hydrophobicity of Src [20]. Ser17 of Src was demonstrated to be phosphorylated by protein kinase A (PKA) in PC12 cells. Src S17A, its dephosphorylated mimic, inhibits Rap1-dependent ERK activation by NGF and cAMP. In addition, neurite outgrowth from PC12 cells by cAMP is also inhibited by Src S17A, suggesting that Ser17 phosphorylation of Src is required for neurite outgrowth by cAMP [21]. In addition to Ser17 phosphorylation by PKA, Thr37 and Ser75 (human; Thr34 and Ser72 in chicken, respectively) and Ser46 (chicken; not found in human) of Src are phosphorylated by Cdk1/Cdc2 during mitosis. Phosphorylations of Thr34, Thr46, and Ser72 residues by p34Cdc2 either sensitize chicken Src to a Tyr527 phosphatase or desensitize Src to a Tyr527 kinase, leading to Src activation [22].
On serine and threonine phosphorylated residues, phosphorylation of Thr37 (human; Thr34 in chicken) and Ser75 (human; Ser72 in chicken) by p25-Cdk5 attenuates lipid binding by the ULBR. Phosphorylation of Ser17, Ser37 and Ser75 of Src gives rise to an electric static repulsion against negative charged lipids within membrane, leading to disruption of Src-membrane interaction [23][24].
4. Acetylation
CREB binding protein (CBP) acetylates the N-terminal lysine residues Lys5, Lys7 and Lys9 of Src to promote dissociation from the cell membrane. In addition, CBP also acetylates the C-terminal Lys401, Lys423, and Lys427 of Src to activate intrinsic kinase activity for STAT3 recruitment and activation, resulting in N-terminal domain phosphorylation (Tyr14, Tyr45, and Tyr68) of STAT3. These phosphorylations of STAT3 lead to formation of STAT3 dimer, an active transcription factor, which translocates to nucleus and then regulates transcription of specific genes there.
5. Ubiquitylation
Loss of Csk reduces Src and the related SFK member, Fyn, protein levels. This is due to Csk stabilizing Src levels, along with inhibition of proteasome activity also increasing Src protein levels in Csk-deficient cells, pointing to protein degradation by a proteasome as the possible mechanism in this regulation. Tyr419-phosphorylated Src, an active form, can undergo Cullin-5-dependent ubiquitylation and activation of Src increases the extent of its polyubiquitylation [25]. Moreover, phosphorylation of Ser75 by Cdk5 promotes the ubiquitin-dependent degradation of Src [26]. Interestingly, ubiquitylated Src at Lys429 is crucial for Src secretion via extracellular vesicles. Mutation of Src at Lys429 also activates the tyrosine kinase FAK to potentiate Src-induced invasive phenotypes [27]. Ubiquitin ligase subunit, FBXL7, mediates the ubiquitylation and proteasomal degradation of active Src after phosphorylation of Src Ser104 residue. It should be noted that the promoter of FBXL7 is hypermethylated in advanced prostate and pancreatic cancers along with decreased mRNA and protein levels of FBXL7 [28].
6. SUMOylation
Src can be SUMOylated at Lys318 in response to hydrogen peroxide. In contrast, hypoxia attenuates Src SUMOylation, along with an increase in Src Y419 phosphorylation. Ectopic expression of SUMO-defective mutant, Src K318R, promotes tumorigenesis. In addition, Src SUMOylation decreases Y925 phosphorylation of tyrosine kinase FAK residue, leading to reduced cell migration. Consequently, SUMOylation of Src at Lys318 negatively modulates its oncogenic function by at least partially inhibiting Src-FAK complex activity [29].
7. Oxidation
Intramolecular disulfide bridge formation between Cys245 and Cys487 upon exposure to ROS leads to Src activation [30]. In contrast, intermolecular disulfide bridges between the Cys277 residues of two different Src proteins result in inactive Src dimers [31]. Src family kinases localized to focal adhesion and the plasma membrane are rapidly and permanently inactivated by hydrogen peroxide. Surprisingly, the levels of cytoplasmic Src family kinases also gradually rise by hydrogen peroxide, in human aortic endothelial cells (HAECs) and human umbilical vein endothelial cells (HUVECs) [32]. All the post-translational modifications of Src are summarized in Figure 2.
Table 1. Post-translational modifications of Src.
| Posttranslational Modification |
Description | References |
|---|---|---|
| Lipidation | Myristic acid is covalently linked to Gly2. | [2] |
| The first 14 amino acids of Src contain a recognition sequence for myristoylation of Src | [3] | |
| The initiating Met is cleaved by methionine amino peptidase and Gly2 become to N-terminal amino acid. | [4] | |
| T456A mutation of Src undergoes detached from membrane, suggesting Thr456 is localized in binding pocket and regulates myristoyl switch. | [5] | |
| N-terminal myristoylated SH4 domain interact with SH3 domain when Src in not anchored to a lipid membrane. | [6] | |
| Myristoyl group is particularly essential for high affinity binding to the lipid bilayer via electrostatic interaction with acidic phospholipid in vitro | [7] | |
| SFKs in N-terminal domain and cysteine(s) can be covalently linked to palmitic acid by palmitoyl acyl transferase (PAT). | [4] | |
| Phosphorylation | Tyr416 ensures an active form. | [8] |
| Tyr527 in C-terminal domain reveals inactivation. | [9] | |
| The Tyr527 phosphorylation is carried out by C-terminal Src kinase. | [10] | |
| The Tyr527 phosphorylation is carried out by its homolog Csk homologous kinase (Chk). | [11][12] | |
| Tyr527 of Src promotes assembly SH2 domain of Src, resulting in intramolecular auto-inhibitory Src structure. | [13][14] | |
| PDGF phosphorylates Tyr213 which interferes with p-Tyr527 binding to SH2 domain in Src leading Src activation. | [17] | |
| Three-dimensional structure of Src with phosphorylations of Tyr416 and Tyr527. | [18] | |
| Phosphorylation/dephosphorylation observed in the Unique domain of Src plays a critical regulatory function in Src kinase activity. | [19] | |
| Ser17 of Src was postulated to be a candidate of phosphorylation residue, which likely changes hydrophobicity of Src. | [20] | |
| Ser17 of Src was demonstrated to be phosphorylated by PKA in PC12 cells. Src S17A (dephospho-mimic) inhibits Rap1-dependent ERK activation by NGF and cAMP. | [21] | |
| The phosphorylations of the Thr34, Thr46, and Ser72 residues by p34Cdc2 either sensitize chicken Src to a Tyr527 phosphatase or desensitize Src to a Tyr527 kinase, leading to Src activation. | [22] | |
| The phosphorylation of Ser37 and Ser75 by p25-Cdk5 attenuates lipid binding by the ULBR. | [23][24] | |
| Ser109 phosphorylation of Src undergoes its degradation. | [28] | |
| Wnt3A induces phosphorylation of Src at Ser43, Ser51 and Ser493 residues through p-Ser9 GSK-3β. | [33] | |
| Acetylation | CREB binding protein (CBP) acetylates N-terminal lysine residues (K5, K7 and K9) of c-Src to promote dissociation from the cell membrane. In addition, CBP also acetylates the c-terminal K401, K423, and K427 of c-Src. | [34] |
| Low aggressive osteosarcoma SaOS-2 cells show high level of Src in nucleus. High metastatic 143B osteosarcoma cells present low levels of nuclear Src. | [35] | |
| EGF induces SRC activation, which phosphorylates Tyr845 in EGFR, resulting in mitochondrial localization of Src and EGFR. | [36] | |
| In mitochondria, EGFR binds to cytochrome C oxidase subunit II (CoxII), and EGFR and Src phosphorylate CoxII, leading to decreases of complex IV activity and ATP levels. | [37] | |
| Src is sequestered to mitochondria with AKAP121. | [38] | |
| Overexpression of the downstream of kinase-4 (Dok-4) containing N-terminal mitochondrial targeting sequence increases mitochondrial Src localization through the complex formation with Src. | [39] | |
| Mitochondrial Src is high in breast cancer cells of triple negative subtype, and targets to phosphorylate mitochondrial single stranded DNA-binding protein (SSBP1), a regulator of mtDNA replication. | [40] | |
| Ubiquitylation | p-Tyr419 Src, an active form can undergo Cullin-5-dependent ubiquitylation and activation of Src increases the extent of polyubiquitylation. | [25] |
| Phosphorylation at Ser75 by Cdk5 promotes the ubiquitin-dependent degradation of Src. | [26] | |
| Mutation at Lys429 activates FAK to potentiate Src-induced invasive phenotypes. | [27] | |
| The promoter of FBXL7 is hypermethylated in advanced prostate and pancreatic cancers along with decreased mRNA and protein levels of FBXL7. | [28] | |
| SUMOylation | SUMOylation of Src at K318 negatively modulates its oncogenic function by at least partially, inhibiting Src-FAK complex activity. | [29] |
| Oxidation | Intramolecular disulfide bridge between Cys245 and Cys487 upon exposure to ROS lead to Src activation. | [30] |
| Intermolecular disulfide bridges between Cys277 residues of two different Src proteins result in inactive Src dimers. | [31] | |
| Cytoplasmic Src family kinases are activated gradually by hydrogen peroxide in human aortic endothelial cells (HAECs) and human umbilical vein endothelial cells (HUVECs). | [32] |
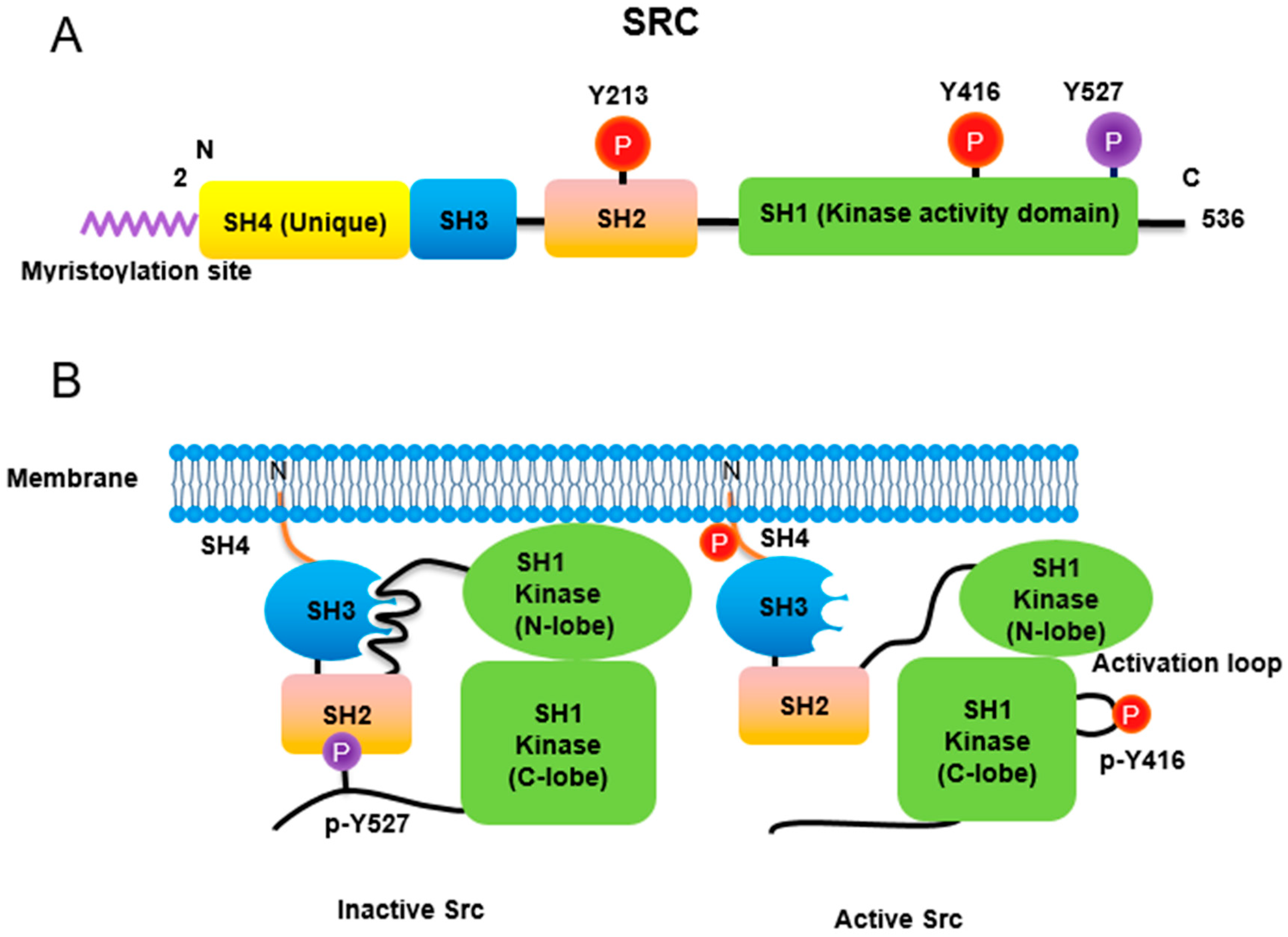
Figure 2. Post-translational modifications of Src. Src undergoes post-translational modification with lipidation, phosphorylation at Ser/Thr and Tyr residues, acetylation, ubiquitylation, SUMOylation and oxidation for its own particular purpose. The numbers of modified amino acid residues in the domains of chicken (human) proteins are denoted. * Referred to chicken only, ** referred to MDCK cell line (Canis familiaris, dog) [27].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10051112
References
- Bagnato, G.; Leopizzi, M.; Urciuoli, E.; Peruzzi, B. Nuclear Functions of the Tyrosine Kinase Src. Int. J. Mol. Sci. 2020, 21, 2675.
- Pellman, D.; Garber, E.A.; Cross, F.R.; Hanafusa, H. An N-terminal peptide from p60src can direct myristylation and plasma membrane localization when fused to heterologous proteins. Nature 1985, 314, 374–377.
- Kamps, M.P.; Buss, J.E.; Sefton, B.M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc. Natl. Acad. Sci. USA 1985, 82, 4625–4628.
- Resh, M.D. Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1999, 1451, 1–16.
- Patwardhan, P.; Resh, M.D. Myristoylation and membrane binding regulate c-Src stability and kinase activity. Mol. Cell Biol. 2010, 30, 4094–4107.
- Le Roux, A.L.; Mohammad, I.L.; Mateos, B.; Arbesu, M.; Gairi, M.; Khan, F.A.; Teixeira, J.M.C.; Pons, M. A Myristoyl-Binding Site in the SH3 Domain Modulates c-Src Membrane Anchoring. iScience 2019, 12, 194–203.
- Sigal, C.T.; Zhou, W.; Buser, C.A.; McLaughlin, S.; Resh, M.D. Amino-terminal basic residues of Src mediate membrane binding through electrostatic interaction with acidic phospholipids. Proc. Natl. Acad. Sci. USA 1994, 91, 12253–12257.
- Smart, J.E.; Oppermann, H.; Czernilofsky, A.P.; Purchio, A.F.; Erikson, R.L.; Bishop, J.M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc. Natl. Acad. Sci. USA 1981, 78, 6013–6017.
- Cooper, J.A.; King, C.S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol. Cell Biol. 1986, 6, 4467–4477.
- Nada, S.; Okada, M.; MacAuley, A.; Cooper, J.A.; Nakagawa, H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 1991, 351, 69–72.
- Hamaguchi, I.; Yamaguchi, N.; Suda, J.; Iwama, A.; Hirao, A.; Hashiyama, M.; Aizawa, S.; Suda, T. Analysis of CSK homologous kinase (CHK/HYL) in hematopoiesis by utilizing gene knockout mice. Biochem. Biophys. Res. Commun. 1996, 224, 172–179.
- Davidson, D.; Chow, L.M.; Veillette, A. Chk, a Csk family tyrosine protein kinase, exhibits Csk-like activity in fibroblasts, but not in an antigen-specific T-cell line. J. Biol. Chem. 1997, 272, 1355–1362.
- Weijland, A.; Williams, J.C.; Neubauer, G.; Courtneidge, S.A.; Wierenga, R.K.; Superti-Furga, G. Src regulated by C-terminal phosphorylation is monomeric. Proc. Natl. Acad. Sci. USA 1997, 94, 3590–3595.
- Xu, W.; Harrison, S.C.; Eck, M.J. Three-dimensional structure of the tyrosine kinase c-Src. Nature 1997, 385, 595–602.
- Su, J.; Muranjan, M.; Sap, J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 1999, 9, 505–511.
- Ponniah, S.; Wang, D.Z.; Lim, K.L.; Pallen, C.J. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 1999, 9, 535–538.
- Stover, D.R.; Furet, P.; Lydon, N.B. Modulation of the SH2 binding specificity and kinase activity of Src by tyrosine phosphorylation within its SH2 domain. J. Biol. Chem. 1996, 271, 12481–12487.
- Boggon, T.J.; Eck, M.J. Structure and regulation of Src family kinases. Oncogene 2004, 23, 7918–7927.
- Amata, I.; Maffei, M.; Pons, M. Phosphorylation of unique domains of Src family kinases. Front. Genet. 2014, 5, 181.
- Walker, F.; deBlaquiere, J.; Burgess, A.W. Translocation of pp60c-src from the plasma membrane to the cytosol after stimulation by platelet-derived growth factor. J. Biol. Chem. 1993, 268, 19552–19558.
- Obara, Y.; Labudda, K.; Dillon, T.J.; Stork, P.J. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J. Cell Sci. 2004, 117, 6085–6094.
- Shenoy, S.; Chackalaparampil, I.; Bagrodia, S.; Lin, P.H.; Shalloway, D. Role of p34cdc2-mediated phosphorylations in two-step activation of pp60c-src during mitosis. Proc. Natl. Acad. Sci. USA 1992, 89, 7237–7241.
- Perez, Y.; Gairi, M.; Pons, M.; Bernado, P. Structural characterization of the natively unfolded N-terminal domain of human c-Src kinase: Insights into the role of phosphorylation of the unique domain. J. Mol. Biol. 2009, 391, 136–148.
- Perez, Y.; Maffei, M.; Igea, A.; Amata, I.; Gairi, M.; Nebreda, A.R.; Bernado, P.; Pons, M. Lipid binding by the Unique and SH3 domains of c-Src suggests a new regulatory mechanism. Sci. Rep. 2013, 3, 1295.
- Hakak, Y.; Martin, G.S. Ubiquitin-dependent degradation of active Src. Curr. Biol. 1999, 9, 1039–1042.
- Pan, Q.; Qiao, F.; Gao, C.; Norman, B.; Optican, L.; Zelenka, P.S. Cdk5 targets active Src for ubiquitin-dependent degradation by phosphorylating Src(S75). Cell Mol. Life Sci. 2011, 68, 3425–3436.
- Tanaka, K.; Ito, Y.; Kajiwara, K.; Nada, S.; Okada, M. Ubiquitination of Src promotes its secretion via small extracellular vesicles. Biochem. Biophys. Res. Commun. 2020, 525, 184–191.
- Moro, L.; Simoneschi, D.; Kurz, E.; Arbini, A.A.; Jang, S.; Guaragnella, N.; Giannattasio, S.; Wang, W.; Chen, Y.A.; Pires, G.; et al. Epigenetic silencing of the ubiquitin ligase subunit FBXL7 impairs c-SRC degradation and promotes epithelial-to-mesenchymal transition and metastasis. Nat. Cell Biol. 2020, 22, 1130–1142.
- Wang, J.; Deng, R.; Cui, N.; Zhang, H.; Liu, T.; Dou, J.; Zhao, X.; Chen, R.; Wang, Y.; Yu, J.; et al. Src SUMOylation Inhibits Tumor Growth Via Decreasing FAK Y925 Phosphorylation. Neoplasia 2017, 19, 961–971.
- Giannoni, E.; Buricchi, F.; Raugei, G.; Ramponi, G.; Chiarugi, P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell Biol. 2005, 25, 6391–6403.
- Kemble, D.J.; Sun, G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc. Natl. Acad. Sci. USA 2009, 106, 5070–5075.
- Tang, H.; Hao, Q.; Rutherford, S.A.; Low, B.; Zhao, Z.J. Inactivation of SRC family tyrosine kinases by reactive oxygen species in vivo. J. Biol. Chem. 2005, 280, 23918–23925.
- Kim, J.G.; Mahmud, S.; Min, J.K.; Lee, Y.B.; Kim, H.; Kang, D.C.; Park, H.S.; Seong, J.; Park, J.B. RhoA GTPase phosphorylated at tyrosine 42 by src kinase binds to beta-catenin and contributes transcriptional regulation of vimentin upon Wnt3A. Redox Biol. 2021, 40, 101842.
- Huang, C.; Zhang, Z.; Chen, L.; Lee, H.W.; Ayrapetov, M.K.; Zhao, T.C.; Hao, Y.; Gao, J.; Yang, C.; Mehta, G.U.; et al. Acetylation within the N- and C-Terminal Domains of Src Regulates Distinct Roles of STAT3-Mediated Tumorigenesis. Cancer Res. 2018, 78, 2825–2838.
- Urciuoli, E.; Coletta, I.; Rizzuto, E.; De Vito, R.; Petrini, S.; D’Oria, V.; Pezzullo, M.; Milano, G.M.; Cozza, R.; Locatelli, F.; et al. Src nuclear localization and its prognostic relevance in human osteosarcoma. J. Cell Physiol. 2018, 233, 1658–1670.
- Boerner, J.L.; Demory, M.L.; Silva, C.; Parsons, S.J. Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol. Cell Biol. 2004, 24, 7059–7071.
- Demory, M.L.; Boerner, J.L.; Davidson, R.; Faust, W.; Miyake, T.; Lee, I.; Huttemann, M.; Douglas, R.; Haddad, G.; Parsons, S.J. Epidermal growth factor receptor translocation to the mitochondria: Regulation and effect. J. Biol. Chem. 2009, 284, 36592–36604.
- Cardone, L.; Carlucci, A.; Affaitati, A.; Livigni, A.; DeCristofaro, T.; Garbi, C.; Varrone, S.; Ullrich, A.; Gottesman, M.E.; Avvedimento, E.V.; et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol. Cell Biol. 2004, 24, 4613–4626.
- Itoh, S.; Lemay, S.; Osawa, M.; Che, W.; Duan, Y.; Tompkins, A.; Brookes, P.S.; Sheu, S.S.; Abe, J. Mitochondrial Dok-4 recruits Src kinase and regulates NF-kappaB activation in endothelial cells. J. Biol. Chem. 2005, 280, 26383–26396.
- Djeungoue-Petga, M.A.; Lurette, O.; Jean, S.; Hamel-Cote, G.; Martin-Jimenez, R.; Bou, M.; Cannich, A.; Roy, P.; Hebert-Chatelain, E. Intramitochondrial Src kinase links mitochondrial dysfunctions and aggressiveness of breast cancer cells. Cell Death Dis. 2019, 10, 940.
This entry is offline, you can click here to edit this entry!
