Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Stem rust or black rust, caused by Puccinia graminis f. sp. tritici Erikss. and E. Henn. (Pgt), leaf rust or brown rust, caused by Puccinia triticina Erikss. (Pt) and the wheat stripe rust or yellow rust, caused by Puccinia striiformis Westend. f. sp. tritici Erikss. (Pst), is a historically crucial economic disease that occurs in almost all wheat-growing regions worldwide.
- Puccinia striiformis f. sp. tritici
- alternate hosts
- stripe rust of wheat
1. Introduction
Wheat is the most cultivated cereal crop, universal staple food, and a host for many pathogens. The most serious threat to wheat crops is a group of rust fungi causing severe yield losses worldwide [1]. Stem rust or black rust, caused by Puccinia graminis f. sp. tritici Erikss. and E. Henn. (Pgt), leaf rust or brown rust, caused by Puccinia triticina Erikss. (Pt) and the wheat stripe rust or yellow rust, caused by Puccinia striiformis Westend. f. sp. tritici Erikss. (Pst), is a historically crucial economic disease that occurs in almost all wheat-growing regions worldwide [2,3]. The early history of mankind is full of fears and threats to these devastating rust pathogens. Since the discovery of rust pathogens, numerous investigations have been conducted on their life cycles for the management of the diseases caused by these fungi. The tenacity of rust fungi as destructive pathogens throughout the wheat-growing areas in the world is attributed to the special features of the pathogen, for example, the production of a large number of spores, inter and intracontinental wind dissemination, and the ability to change genetically resulting in new races with increased virulence diversity [4]. Generally, the disease occurs in the northern and southern areas of temperate regions. Recently, the wheat stripe rust disease has become more severe in some warmer areas than before [5], endangering global food security [6]. Hot summers and dry seasons are the bottlenecks for the survival of Pst. The disease can be controlled by growing resistant cultivars, suitable cultural practices, and the appropriate use of chemical fungicides. Resistant cultivars are the most effective, economical and environmentally friendly approach to combat with the wheat stripe rust pathogen. However, the Pst population is highly dynamic and variable, which makes it difficult to develop highly resistant wheat cultivars with durable resistance [6].
In the US Pacific Northwest, barberry is essential for the wheat stem rust but does not play a role for the wheat stripe rust pathogen [7,8,9]. Barberry may serve as an alternate host for Pst in the Himalayan region under natural conditions [10,11,12]. In eastern Africa and western Asia, barberry plants have been found, but their association with the wheat stripe rust disease epidemics has not been confirmed [13,14]. To date, the evidence of natural infection of barberry by Pst has been observed only in China, but at a low frequency [15,16]. Similarly, Pst has not been found on barberry plants in southeastern Sweden, but Pgt is common on the alternate host plants in this region [17].
The use of genetic techniques in the past ten years has achieved some advancement in understanding the plant–microbe interaction. The wheat stripe rust pathogen is an obligate, biotrophic parasite, having five distinct spore stages and two hosts to complete their life cycle. A macrocyclic life cycle comprises of uredinial, telial, basidial, pycnial and aecial stages. Like other rust pathogens, Pst is also highly specific to their primary host plants, for example, cereal crops and grasses, and the alternate host plants, for example, Berberis and Mahonia spp. The primary hosts can be the same but generally, the alternate hosts are different for different Puccinia spp. Based on their host specificity and morphological characteristics, the Puccinia spp. are further divided into formae speciales or varieties. For example, stripe rust on wheat is caused by P. striiformis f. sp. tritici (Pst), on barley by P. striiformis f. sp. hordei (Psh); stem rust on wheat by P. graminis f. sp. tritici (Pgt), and on oat by P. graminis f. sp. avenae (Pga), and on rye by P. graminis f. sp. secalis (Pgs); and crown rust on oat by P. coronata var. avenae (Pca), and on barley by P. coronata var. hordei (Pch) [18].
The genetic diversity of Pst in Australia, Europe and North America indicated a clonal population structure of the pathogen [19]. On the other hand, the Pst populations of Gansu Province, China, were found to have high genetic diversities and produce abundant telia, indicating possible sexual recombination in this region [20,21]. Jin et al. [13] reported barberry as an alternate host for P. pseudostriiformis (Syn. P. striiformis f. sp. poae) under natural conditions in Minnesota in the US and Pst under controlled conditions. The possible role of Berberis spp. as a sexual host of Pst has attained much importance, particularly in the US, China, and Pakistan [7,9,11,15,18]. Mahonia aquifolium, under experimental conditions, has also been identified to be susceptible to Pst [22].
2. Stripe Rust: Outlook
Wheat rusts have created major famines throughout history, causing substantial economic losses [37]. Currently, the most severe rust disease is the wheat stripe rust disease, causing more than 60% yield losses under favorable conditions [1,25,38]. The disease is named as yellow rust or stripe rust due to yellow colored stripes in lines between leaf veins in adult-plants but the urediniospores are in clusters (not in stripes) when the infection is at the seedlings stage [39]. Urediniospores are dikaryotic and produced asexually on the primary host plant. In the case of severe disease epidemics, stripe rust uredinial infection occurs on leaves, spikes, spikelets, glumes, awns and kernels. With the increase in temperature or at the maturity stage of the plant, the production of urediniospores comes to an end. The uredinia start converting into black colored telia containing teliospores [40]. Teliospores may serve as the survival structures which can cause infection on the alternate hosts under favorable environmental conditions or become a dead-end due to severe climatic conditions or incompatibility with the alternate host plants [41]. Teliospores are thick walled and germinate to produce haploid basidiospores [18]. These basidiospores directly penetrate the alternate host epidermal cells and cause infection and produce pycnia on the upper side of leaves and aecia on the lower side of leaves. Under favorable conditions both the pycnial and aecial infections are observed on stems, pedicels and peduncles [12]. The aeciospores infect wheat crops resulting in the formation of urediniospores. In contrast to uredinial re-infection on the same primary host plants or grasses, the aeciospores cannot re-infect the alternate host plants.
The wheat stripe rust pathogen belongs to the genus—Puccinia, family—Pucciniaceae, order—Pucciniales, class— Pucciniomycetes, division—Basidiomycota and kingdom—Fungi. Pst can undergo long-distance dispersal and it has caused numerous invasions [42,43] associated with austere economical losses [19,44,45,46]. Several cases of incursions of economic importance have been reported for Pst but only recently their origin was confirmed [11,43]. In the early 20th century, Pst was reported for the first time in South and North America [47,48], most likely spreading from north-western Europe [11,45] and introduced accidentally in Australia from north-western Europe in 1979 through human activity [49]. Pst strains detected in South Africa in 1996 were genetically related to populations in the Mediterranean regions and Middle Eastern ones, possibly spread by the wind [11,50]. Pst has become important in the context of invasions and recolonizations through the emergence of new races and strains in previously non-colonized areas. For example, since 2000, the emergence of two aggressive strains of Pst, PSTS1, and PSTS2, in the geographical expansion of Pst epidemics into the southeastern US and western Australia, where the disease was not previously a serious problem [40,51]. Similarly, since 2011, invasive strains of the wheat stripe rust pathogen, Warrior and Kranich, have largely replaced the pre-existing northwestern European Pst populations of the pathogen [43,52].
Pst has been reported in the areas of the US (Pacific Northwest), eastern Asia (northwestern and southwestern China), Oceania (Australia, New Zealand), southern Asia (India, Pakistan, and Nepal), western Europe (eastern England), the Arabian Peninsula (Yemen) and eastern Africa (Ethiopia, Kenya) [53]. In the last two decades, the emergence of more aggressive races of Pst having the ability to cause high epidemic potential even in warmer regions [39] is generally the result of mutation, somatic hybridization and sexual recombination. The potential role of alternate hosts in pathogenic diversity is of much importance. However, it is still unknown by which mechanisms new races evolve. The high reproduction ability, long distance dissemination, adaptation to different environmental conditions, and several host species make Pst a highly diversified pathogen [54]. The threat of new virulent races of this pathogen emphasizes the need to understand the mechanisms involved in the genetic diversity of Pst and the role of aecial hosts in sexual reproduction to encounter the possible attacks of Pst in the future [39].
3. The Life Cycle of Pst
The complete life cycle of Pst has five spore stages, different from each other, on two phylogenetically distinct host plants, a cereal as the primary host or asexual host, and Berberis spp. as the alternate host or sexual host [13]. The dikaryotic (N + N’) single-celled urediniospores appear on the primary host through the breaking of epidermal cells, and each uredinia harboring yellow-colored numerous urediniospores. The repeated asexual cycles on the primary host may cause wide-scale epidemics on the cereal hosts [55]. The single uredinia, assembled in lesions, forms typical stripes on the leaves of adult plants and produce urediniospores in 10 to 18 days after infection under optimal conditions. The uredinial lesions expand longitudinally upon the production of new uredinia. With the start of the senescence of infected leaves, P. striiformis starts producing telia resulting in the creation of many two-celled oblong-clavate teliospores. These teliospore cells contain a diploid nucleus (N + N’) formed by karyogamy. The germinating teliospores produce ellipsoid haploid (N) basidiospores. Basidiospores are uninucleate or binucleate haploid spores produced from a germinating diploid teliospores. Basidiospores cause infection on the alternate host (e.g., barberry), resulting in oblong-shaped pycniospores (N) on the adaxial surface of the leaf, following the formation of dikaryotic (N + N’) aecia on the abaxial surface of the leaf. Pycniospores are haploid (N), sexually derived spores (spermatium) formed in a pycnium (spermogonium) of rust fungi. Finally, aeciospores infect the primary host resulting urediniospores into the wheat leaves. The life cycle of Pst may take place during two regular growing seasons of the asexual host. Pst is an obligate biotrophic fungus which depends on a living host for its development and reproduction [24,45,55].
The sexual phase starts when the two-celled teliospores germinate and produce basidiospores, attached to a sterigmatum. Basidiospores (N) infect barberry leaves, resulting in the formation of pycnia (N), covered with pycnial nectar, formed on the upper side of the leaf. The haploid pycniospores of (−) and haploid hyphae (+) fuse together through plasmogamy and form aeciospores (N + N) on the abaxial side of the barberry leaf [56]. Aeciospores are dikaryotic produced in a cup-shaped aecium of a rust fungus. The asexual infection process in cereal hosts starts via a urediniospore-germ-tube, penetrating a stoma and then differentiating into a substomatal vesicle, resulting in two to three primary infection hyphae, which develop haustorial mother cells. These cells are separated from their respective hyphae by a septum. Haustorial mother cells penetrate the plant cell walls and form haustoria, highly specialized structures, representing the primary interface between host and the pathogen. Haustoria take water and nutrients from the host tissues and also make signaling between hosts and pathogens by producing effector molecules, like avirulence gene products. Young haustoria have a spherical shape, whereas older haustoria appeared more branched which allows the fungus to extend the area of the contact zone into the host and uptake nutrients more efficiently [45]. Basidiospores can only infect alternate hosts (like barberry) but cannot infect primary hosts (like wheat). Basidiospores infect epidermal cells through direct penetration while urediniospores infect through host stromata. Pst generally infects common wheat (Triticum aestivum L.), cultivated emmer wheat (T. dicoccum Schrank), triticale (Triticosecale), durum wheat (T. turgidum var. durum L.) and wild emmer wheat (T. dicoccoides Korn); as well as cultivated barley (Hordeum vulgare L.) and rye (Secale cereale L.). The complete life cycle of Pst is shown in Figure 1.
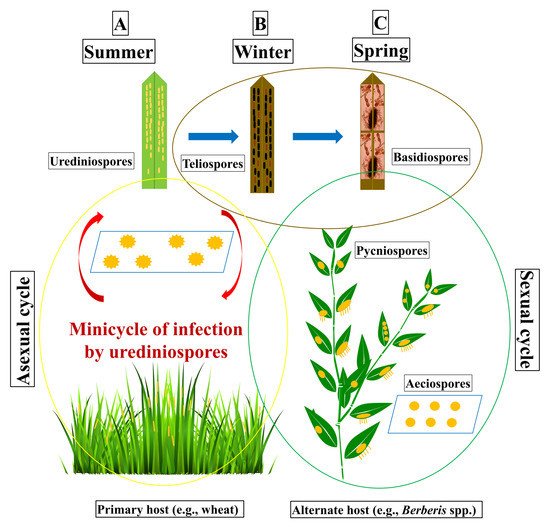
Figure 1. Schematic diagram of the life cycle of Puccinia striiformis f. sp. tritici (Pst), divided into three phases from left to right. Phase A, usually starts late in the spring and continues into the summer until the harvesting of the wheat crop. It consists of the asexual cycle which takes place on the primary host (e.g., wheat). The infection may take place by aeciospores or urediniospores resulting in the formation of yellow-colored stripes of uredinia on the wheat leaves. Urediniospores re-infect wheat plants in the same field or in the neighboring wheat fields to continue the mini-cycle of somatic reproduction until the conditions become unfavorable. With the increase in temperature or at crop maturity, the urediniospores turn into teliospores. Teliospores are thick-walled resting spores. Phase B, the teliospores survive and germinate to produce basidiospores. Phase C, the basidiospores infect the new leaves of alternate hosts (e.g., barberry) in the spring and produce pycniospores on the adaxial surface of leaves. Pycniospores produce aecial cups on the abaxial surface of the leaves after fertilization. Aecial cups contain aeciospores which can infect the primary hosts.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens9060434
This entry is offline, you can click here to edit this entry!
