Psoriasis is associated with increased production of reactive oxygen species which leads to oxidative stress. As antioxidants can provide protection, the aim of this study was to evaluate the effects of cannabidiol (CBD) on neutrophil extracellular trap (NET) formation in psoriatic and healthy neutrophils. Important markers of NETosis were measured in healthy and psoriatic neutrophils after incubation with CBD, lipopolysaccharide (LPS), and LPS + CBD). The percentage of neutrophils undergoing NETosis and the level of NETosis markers (cfDNA, MPO, elastase) were higher in the neutrophils and blood plasma of psoriatic patients, compared to controls. After LPS treatment, all of the markers of NETosis, except elastase, and p47 and citrullinated histones, were increased in samples from healthy subjects and psoriasis patients. CBD reduced the concentrations of NETosis markers. This led to a reduction in NETosis, which was more pronounced in psoriatic neutrophils and neutrophils treated with LPS in both psoriatic and healthy participants. These results suggest that psoriatic patients neutrophils are at a higher risk of NETosis both in vitro and in vivo. CBD reduces NETosis, mainly in psoriatic neutrophils, possibly due to its antioxidant properties. The anti-NET properties of CBD suggest the positive effect of CBD in the treatment of autoimmune diseases.
- NETs
- cannabidiol treatment
- immunity
- neutrophil traps
- psoriasis
1. Introduction
Psoriasis is the most common autoimmune disease and is usually caused by pathological interactions between lymphocytes and keratinocytes in the skin epithelium [1]. Cytokines, produced by lymphocytes, mediate these interactions. Several cytokines are known to be important in psoriasis pathogenesis. Interferon gamma (IFNγ) increases the proliferation of keratinocytes and activates other immune cells [2,3] and interleukin-22 (IL-22) is responsible for increased keratinocyte proliferation [4]. Tumor necrosis factor alpha (TNFα) [5] and interleukin 17 (IL-17) stimulate the production of chemoattractants in keratinocytes. Chemoattractants (hBD2, S100A9, S100A7, S100A, CCL20) increase migration of leukocytes into the epithelium resulting in inflammation. Inhibitors of TNFα and IL-17 are used as therapeutics for psoriasis, demonstrating the importance of these cytokines in pathogenesis [6,7].
Increased inflammation and the over-proliferation of keratinocytes leads to a characteristic skin change called a psoriatic plaque. Psoriatic plaques can lead to psychological and sociological problems that have a strongly negative effect on the patient’s quality of life. While often associated as a disease of the skin, psoriasis may cause systemic issues [1]. The increased production of cytokines can also over-activate circulating lymphocytes and neutrophils present in the blood [7,8]. It has also been shown that psoriatic granulocytes are characterized by higher production of cytokines and reactive oxygen species (ROS) when compared to healthy individuals [8]. In neutrophils, the over-production of ROS induced by NADPH oxidase may lead to a process called NETosis [9].
It was previously shown that during NETosis antimicrobial proteins such as myeloperoxidase (MPO), neutrophil elastase, and cathepsin G are released into the cytoplasm from azurophilic granules within neutrophils and that NETs also contain them [9]. MPO uses superoxides and hydrogen peroxide generated during an oxidative burst to produce hypochlorous acid and other reactive oxidants. Neutrophil elastase migrates into nucleus, where it degrades proteins that are necessary to maintain chromatin structure, such as H1 histones, which results in chromatin decondensation [10]. At the same time, other histones (H2A, H3, H4) undergo citrullination by peptidylarginine deiminase 4, and the nuclear membrane disrupts. When chromatin is released into the cytoplasm, it is attacked by the antimicrobial proteins produced by neutrophils mentioned above. Finally the cell membrane is disrupted and neutrophil extracellular traps (NETs) are formed [11]. These traps are generated from extracellular DNA associated with antimicrobial proteins originating from neutrophil granules and take the form of a net. The traps bind and destroy pathogens. This is possible because the bacterial cells adhere to DNA and the NETs, in effect, limit their spread. A similar mechanism is observed in other leukocytes, as eosinophils, basophils, mast cells and monocytes are also able to form NETs [12,13,14]. Enhanced NETosis is often observed in other autoimmune diseases besides psoriasis including, systemic lupus erythematosus (SLE) and rheumatic arthritis [12,15]. Since the formation of extracellular traps results in the release of all molecules and proteins contained within cells, including various cytokines, NETosis may be a strong inducer of a pro-inflammatory response in autoimmune diseases. For example, in psoriasis, neutrophils are observed to be the most IL-17 positive skin infiltrating cells, indicating they can be an important source of this and other cytokines [15].
Since neutrophils, like other leukocytes in psoriasis, are constantly over-activated due to pro-inflammatory conditions, they may be at greater risk of various processes including NETosis. These cells may be particularly susceptible to NETosis because psoriasis increases the expression of IL-17, which is a potent activator of NETosis [15]. Therefore, modulation of neutrophil function can be a promising treatment for psoriasis, since current therapies are usually ineffective or induce serious side effects. The psoriasis antibody therapies are very expensive, so they are used only in the most severe cases. Therefore, new compounds are being sought, especially of natural origin, as potential drugs in the treatment of psoriasis.
One potential candidate for therapeutic action seems to be cannabidiol (CBD)—a phytocannabinoid found in Cannabis Sativa L., which has no psychoactive effect, but is an anti-inflammatory and antioxidant compound, and its use has shown a positive effect on psoriatic skin cells [16,17]. Since it is known that CBD may reduce neutrophil activity and ROS production therein [18,19], its beneficial effect may be, at least in part, due to the weakening of the skin infiltrating neutrophils. By preventing the production of ROS, CBD may act as a NETosis inhibitor.
2. Results
The results show that the percentage of unstimulated neutrophils undergoing NETosis was greater in the psoriasis vulgaris cohort than in healthy control subjects (Figure 1). Incubation with LPS, a known activator of NETosis, increased the number of neutrophils undergoing NETosis in both groups, although the concentration of NETosis was greater in the psoriasis cohort. Treatment with CBD did not cause significant decrease in the number of NETotic cells compared to untreated. In contrast, activation of neutrophils with LPS in conjunction with CBD significantly reduced the percentage of neutrophils undergoing NETosis compared to treatment with LPS alone.
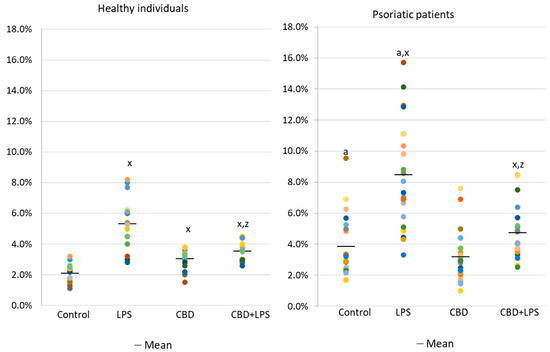
Figure 1. Percentage of neutrophils from healthy subjects (n = 14) and psoriatic patients (n = 28) undergoing NETosis. after 1h incubation with lipopolysaccharide (LPS), cannabidiol (CBD) and LPS with cannabidiol. a—statistically significant difference between neutrophils from patients with psoriasis vulgaris and healthy subjects p < 0.05; x—statistically significant difference between treated neutrophils (with LPS, CBD, or LPS + CBD) and non-treated (control) cells; p < 0.05; z—statistically significant difference between neutrophils treated with LPS + CBD and treated only with LPS; p < 0.05.
Neutrophils undergoing NETosis show metabolic changes in these cells, assessed by the level of various parameters. The production of the p47 subunit of prooxidative NADPH oxidase, responsible for the generation of superoxide radicals, is strongly increased after LPS administration, especially in neutrophils of psoriatic patients (Figure 2). In this case, CBD appears to enhance p47 production in LPS-treated neutrophils of healthy subjects but does not affect production in psoriasis neutrophils. In neutrophils treated only with CBD decreases in production of p47 was observed in psoriatic patients. In summary, the results suggest that CBD may act as an activator of pro-oxidative NADH of neutrophils in healthy people, but it does not intensify the already existing pro-oxidative conditions in neutrophils from patients with psoriasis.
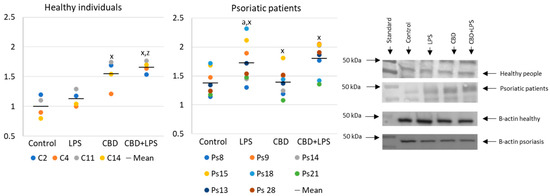
Figure 2. The level of p47 in neutrophils from heathy subjects (n = 4) and psoriatic patients (n = 8) after 1 h incubation with LPS, cannabidiol and LPS with cannabidiol. a—statistically significant difference between neutrophils from patients with psoriasis vulgaris and healthy subjects p < 0.05; x—statistically significant difference between treated neutrophils (with LPS, CBD, or LPS + CBD) and non-treated (control) cells; p < 0.05; z—statistically significant difference between neutrophils treated with LPS + CBD and treated only with LPS; p < 0.05.
In contrast to p47, MPO is released from the cell during NETosis and is therefore assessed in supernatants rather than intracellularly (Figure 3). Since MPO is one of the important markers of NETosis, higher concentration in supernatants of neutrophils obtained from psoriasis patients and in LPS-treated cells suggests an increased NETosis in these groups. On the other hand, in the case of cells treated with CBD, the MPO level is lower, which may inhibit the generation of hypochlorous acid and indicate a decrease in NETosis. Additionally, CBD has an anti-NETotic effect in LPS-treated cells.
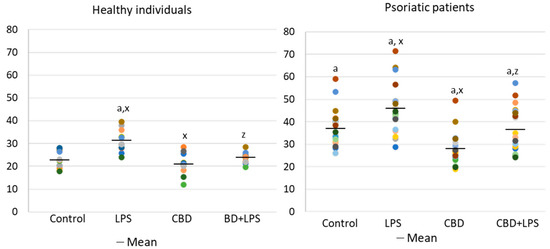
Figure 3. Level of myeloperoxidase (MPO) (ng/mL) in supernatants obtained after 1h neutrophils from healthy subjects (n = 14) and psoriatic patients (n = 28) incubation with LPS, cannabidiol and LPS with cannabidiol. a—statistically significant difference between neutrophils from psoriasis vulgaris and healthy subjects p < 0.05; x—statistically significant difference between treated neutrophils (with LPS, CBD, or LPS + CBD) and non-treated (control) cells; p < 0.05; z—statistically significant difference between neutrophils treated with LPS + CBD and treated only with LPS; p < 0.05.
Changes in the generation or activity of pro-oxidative proteins in NETosis are also accompanied by histone citrullination (Figure 4). In this case, LPS led to the activation of NETosis, which resulted in increased expression of citrullinated H3-histones. On the other hand, CBD showed anti-NETotic properties, especially in relation to the psoriatic and LPS-activated neutrophils.
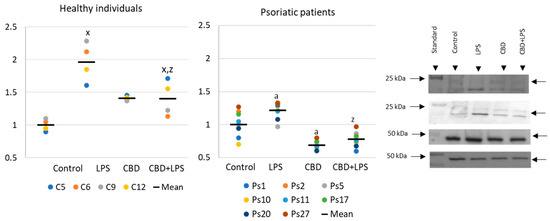
Figure 4. Citrullinated H3-histone expression in human neutrophils from heathy subjects (n = 4) and psoriatic patients (n = 8) after 1 h incubation with LPS, cannabidiol and LPS with cannabidiol. a—statistically significant difference between neutrophils from patients with psoriasis vulgaris and healthy subjects p < 0.05; x—statistically significant difference between treated neutrophils (with LPS, CBD, or LPS + CBD) and non-treated (control) cells; p < 0.05; z—statistically significant difference between neutrophils treated with LPS + CBD and treated only with LPS; p < 0.05.
Increased production of neutrophilic elastase is necessary for NETosis and production of elastase was much greater in neutrophils of patients with psoriasis than in healthy subjects, suggesting increased activation of NETosis. CBD appears to have an anti-inflammatory effect as it leads to a reduction in the production of elastase in neutrophils of psoriasis patients. It can therefore be suggested that CBD reduces the effect of LPS on plaque neutrophils (Figure 5).
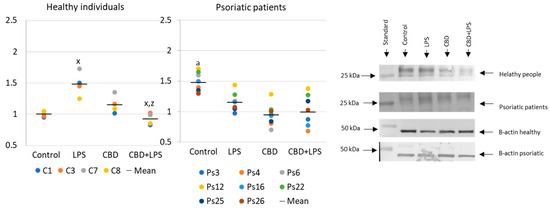
Figure 5. Neutrophil elastase expression in human neutrophils from heathy subjects (n = 4) and psoriatic patients (n = 8) after 1 h incubation with LPS, cannabidiol and LPS with cannabidiol. a—statistically significant difference between neutrophils from patients with psoriasis vulgaris and healthy subjects p < 0.05; x—statistically significant difference between treated neutrophils (with LPS, CBD, or LPS + CBD) and non-treated (control) cells; p < 0.05; z—statistically significant difference between neutrophils treated with LPS + CBD and treated only with LPS; p < 0.05.
Since the action of the described enzymes ultimately leads to the release of the DNA, cfDNA it is one of the most reliable markers of NETosis. In the present study, an increase in the concentration of cfDNA was observed in supernatants obtained after incubation of neutrophils from psoriasis patients as well as after incubation of neutrophils with LPS. On the other hand, CBD leads to a reduction in cfDNA levels, suggesting that the effect of CBD on neutrophils ultimately decreases NETosis (Figure 6).
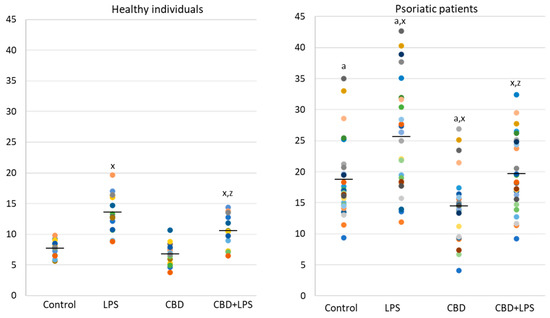
Figure 6. Level of cfDNA (ng/mL) in supernatants obtained after 1h neutrophils, from healthy subjects (n = 14) and psoriatic patients (n = 28), incubation with LPS, cannabidiol and LPS with cannabidiol. a—statistically significant difference between neutrophils from patients with psoriasis vulgaris and healthy subjects p < 0.05; x—statistically significant difference between treated neutrophils (with LPS, CBD, or LPS + CBD) and non-treated (control) cells; p < 0.05; z—statistically significant difference between neutrophils treated with LPS + CBD and treated only with LPS; p < 0.05.
As the intensity of NETosis is dependent on the activity of neutrophils, it may be also dependent on the severity of psoriasis. Correlations between the Psoriasis Area Severity Index (PASI)—the most important marker used in clinical practice for the assessment of psoriasis—and in vitro markers of NETosis show that in most cases severity of psoriasis correlates with disease severity (Figure 7). Moreover, it was observed that correlations are still clear also after administration of LPS or CBD in case of percent of neutrophils undergoing NETosis and MPO level. On contrary level of cfDNA correlates with neutrophils stimulated with LPS and LPS + CBD.
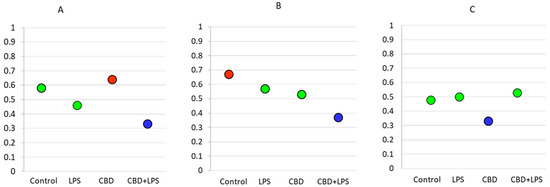
Figure 7. Spearman’s correlation index between PASI and relevant parameters. Blue circle—low correlation (Spearman correlation below 0.4); Green circle—medium correlation (Spearman correlation index from 0.4 to 0.6); Red circle—high correlation (Spearman correlation index from 0.6 to 0.8); (A) Percentage of NETotic cells and PASI; (B) MPO level and PASI; (C) cfDNA and PASI.
As mentioned, both MPO and cfDNA are externalized outside the cells, therefore they can also be measured directly in blood plasma, and the level of these parameters may indicate the intensification of NETosis. ELISA assays showed that cfDNA and MPO concentrations are significantly higher in psoriatic patients than healthy subjects, suggesting an increase in NETosis in these patients (Figure 8).
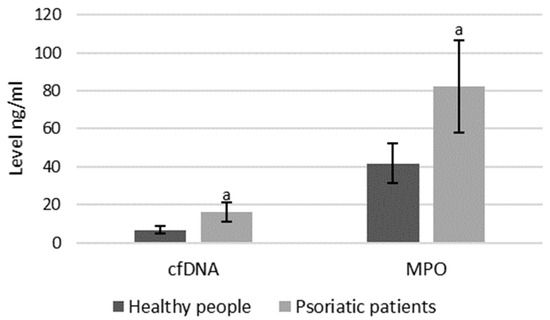
Figure 8. Plasma MPO and cfDNA levels in healthy subjects (n = 14) and patients with psoriasis (n = 28). a—statistically significant difference between neutrophils from patients with psoriasis vulgaris and healthy subjects; p < 0.05.
References
- Rendn, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475.
- Kryczek, I.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Aphale, A.; Vatan, L.; Szeliga, W.; Wang, Y.; Liu, Y.; Welling, T.H.; et al. Induction of IL-17+ T cell trafficking and development by IFN-γ: Mechanism and pathological relevance in psoriasis. J. Immunol. 2008, 181, 4733–4741.
- Cai, Y.; Fleming, C.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol. Immunol. 2012, 9, 302–309.
- Fujita, H. Role of IL-22 in the pathogenesis of skin diseases. Nihon Rinsho Meneki Gakkai Kaishi 2012, 35, 168–175.
- Fantuzzi, F.; Giglio, M.D.; Gisondi, P.; Girolomoni, G. Targeting tumor necrosis factor α in psoriasis and psoriatic arthritis. Expert Opin. Ther. Targets 2008, 12, 1085–1096.
- Chaudhari, U.; Romano, P.; Mulcahy, L.D.; Dooley, L.T.; Baker, D.G.; Gottlieb, A.B. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: A randomised trial. Lancet 2001, 357, 1842–1847.
- Wójcik, P.; Biernacki, M.; Wroński, A.; Łuczaj, W.; Waeg, G.; Žarković, N.; Skrzydlewska, E. Altered Lipid Metabolism in Blood Mononuclear Cells of Psoriatic Patients Indicates Differential Changes in Psoriasis Vulgaris and Psoriatic Arthritis. Int. J. Mol. Sci. 2019, 20, 4249.
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159.
- Rada, B. Neutrophil Extracellular Traps. Methods Mol. Biol. 2019, 1982, 517–528.
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691.
- Remijsen, Q.; Vanden Berghe, T.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304.
- Lee, K.H.; Kronbichler, A.; Park, D.D.-Y.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173.
- Möllerherm, H.; von Köckritz-Blickwede, M.; Branitzki-Heinemann, K. Antimicrobial Activity of Mast Cells: Role and Relevance of Extracellular DNA Traps. Front. Immunol. 2016, 7.
- Schorn, C.; Janko, C.; Latzko, M.; Chaurio, R.; Schett, G.; Herrmann, M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front. Immunol. 2012, 3.
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500.
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92.
- Palmieri, B.; Laurino, C.; Vadalà, M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin. Ter. 2019, 170, e93–e99.
- Mabou Tagne, A.; Marino, F.; Legnaro, M.; Luini, A.; Pacchetti, B.; Cosentino, M. A Novel Standardized Cannabis sativa L. Extract and Its Constituent Cannabidiol Inhibit Human Polymorphonuclear Leukocyte Functions. Int. J. Mol. Sci. 2019, 20, 1833.
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanashian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21186795
