Childhood cancer is a rarely occurring disease. However, it is the second most frequent cause of death among children. It is estimated that each year cancer will be diagnosed in 400,000 children worldwide. The nutritional status at diagnosis is a prognostic indicator and influences the treatment tolerance. Both malnutrition and obesity increase the risk of mortality and complications during treatment. It is necessary to constantly search for new factors that impair the nutritional status. The endocannabinoid system (ECS) is a signaling system whose best-known function is regulating energy balance and food intake, but it also plays a role in pain control, embryogenesis, neurogenesis, learning, and the regulation of lipid and glucose metabolism. Its action is multidirectional, and its role is being discovered in an increasing number of diseases.
- cancer
- children
- nutritional status
- endocannabinoid system
1. Nutritional Status in Children with Cancer
1.1. Nutritional Disorders in Children Diagnosed with Various Types of Cancer
1.1.1. Leukemia
1.1.2. Solid Tumors
1.1.3. Central Nervous System (CNS) Tumors
1.2. Bone Health in Children with Cancer
2. Regulation of Appetite in Children with Cancer
2.1. Appetite Regulation
2.2. The Causes of Appetite and Nutritional Status Disorders in Children with Cancer
3. The Role of Endocannabinoids System in Childhood Cancer
3.1. Physiology of ECS
3.2. The Role of the ECS in the Regulation of Appetite
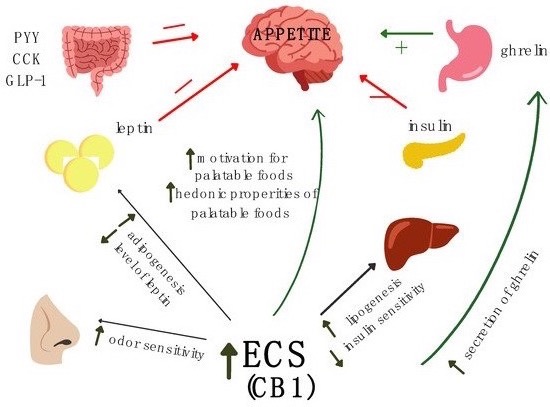
3.3. The Role of ECS in Childhood Cancer
This entry is adapted from the peer-reviewed paper 10.3390/ijms23095159
References
- Barr, R.D.; Stevens, M.C.G. The influence of nutrition on clinical outcomes in children with cancer. Pediatr. Blood Cancer 2020, 67, e28117.
- Viani, K.; Trehan, A.; Manzoli, B.; Schoeman, J. Assessment of nutritional status in children with cancer: A narrative review. Pediatr. Blood Cancer 2020, 67, e28211.
- Rogers, P.C. Nutritional status as a prognostic indicator for pediatric malignancies. J. Clin. Oncol. 2014, 32, 1293–1294.
- Brinksma, A.; Huizinga, G.; Sulkers, E.; Kamps, W.; Roodbol, P.; Tissing, W. Malnutrition in childhood cancer patients: A review on its prevalence and possible causes. Crit. Rev. Oncol. Hematol. 2012, 83, 249–275.
- Triarico, S.; Rinninella, E.; Cintoni, M.; Capozza, M.A.; Mastrangelo, S.; Mele, M.C.; Ruggiero, A. Impact of malnutrition on survival and infections among pediatric patients with cancer: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1165–1175.
- Diakatou, V.; Vassilakou, T. Nutritional Status of Pediatric Cancer Patients at Diagnosis and Correlations with Treatment, Clinical Outcome and the Long-Term Growth and Health of Survivors. Children 2020, 7, 218.
- Iniesta, R.R.; Paciarotti, I.; Davidson, I.; McKenzie, J.M.; Brougham, M.F.H.; Wilson, D.C. Nutritional status of children and adolescents with cancer in Scotland: A prospective cohort study. Clin. Nutr. ESPEN 2019, 32, 96–106.
- Pribnow, A.K.; Ortiz, R.; Báez, L.F.; Mendieta, L.; Luna-Fineman, S. Effects of Malnutrition on Treatment-Related Morbidity and Survival of Children with Cancer in Nicaragua. Pediatr. Blood Cancer 2017, 64, e26590.
- Iniesta, R.R.; Paciarotti, I.; Brougham, M.F.; McKenzie, J.M.; Wilson, D.C. Effects of pediatric cancer and its treatment on nutritional status: A systematic review. Nutr. Rev. 2015, 73, 276–295.
- Brinksma, A.; Roodbol, P.F.; Sulkers, E.; Kamps, W.A.; de Bont, E.S.; Boot, A.M.; Burgerhof, J.G.; Tamminga, R.Y.; Tissing, W.J. Changes in nutritional status in childhood cancer patients: A prospective cohort study. Clin. Nutr. 2015, 34, 66–73.
- Paciarotti, I.; McKenzie, J.M.; Davidson, I. Short term effects of childhood cancer and its treatments on nutritional status: A prospective cohort study. EC Nutr. 2015, 3, 528–540.
- World Health Organization. CureAll Framework: WHO Global Initiative for Childhood Cancer: Increasing Access, Advancing Quality, Saving Lives. License: CC BY-NC-SA 3.0 IGO. 2021. Available online: https://apps.who.int/iris/handle/10665/347370 (accessed on 11 January 2021).
- Lange, B.J.; Gerbing, R.B.; Feusner, J.; Skolnik, J.; Sacks, N.; Smith, F.O.; Alonzo, T.A. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA 2005, 12, 203–211.
- Orgel, E.; Genkinger, J.M.; Aggarwal, D.; Sung, L.; Nieder, M.; Ladas, E.J. Association of body mass index and survival in pediatric leukemia: A meta-analysis. Am. J. Clin. Nutr. 2016, 103, 808–817.
- Saenz, A.M.; Stapleton, S.; Hernandez, R.G.; Hale, G.A.; Goldenberg, N.A.; Schwartz, S.; Amankwah, E.K. Body Mass Index at Pediatric Leukemia Diagnosis and the Risks of Relapse and Mortality: Findings from a Single Institution and Meta-Analysis. J. Obes. 2018, 2018, 7048078.
- Amankwah, E.K.; Saenz, A.M.; Hale, G.A.; Brown, P.A. Association between body mass index at diagnosis and pediatric leukemia mortality and relapse: A systematic review and meta-analysis. Leuk. Lymphoma 2016, 57, 1140–1148.
- Butturini, A.M.; Dorey, F.J.; Lange, B.J.; Henry, D.W.; Gaynon, P.S.; Fu, C.; Franklin, J.; Siegel, S.E.; Seibel, N.L.; Rogers, P.C.; et al. Obesity and Outcome in Pediatric Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2007, 20, 2063–2069.
- Orgel, E.; Sposto, R.; Malvar, J.; Seibel, N.L.; Ladas, E.; Gaynon, P.S.; Freyer, D.R. Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J. Clin. Oncol. 2014, 1, 1331–1337.
- Orgel, E.; Tucci, J.; Alhushki, W.; Malvar, J.; Sposto, R.; Fu, C.H.; Freyer, D.R.; Abdel-Azim, H.; Mittelman, S.D. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood 2014, 124, 3932–3938.
- Hijiya, N.; Panetta, J.C.; Zhou, Y.; Kyzer, E.P.; Howard, S.C.; Jeha, S.; Razzouk, B.I.; Ribeiro, R.C.; Rubnitz, J.E.; Hudson, M.M.; et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood 2006, 108, 3997–4002.
- Browne, E.K.; Jeha, S.; Cheng, C.; Relling, M.V.; Campana, D.; Pui, C.H.; Inaba, H. The effect of body mass index at diagnosis on clinical outcome in children with newly diagnosed acute lymphoblastic leukemia. Blood Cancer J. 2017, 7, e531.
- Aldhafiri, F.K.; McColl, J.H.; Reilly, J.J. Prognostic significance of being overweight and obese at diagnosis in children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2014, 36, 234–236.
- Jaime-Pérez, J.C.; Turrubiates-Hernández, G.A.; García-Salas, G.; de la Torre-Salinas, A.M.; Áncer-Rodríguez, P.; Villarreal-Martínez, L.; Gómez-Almaguer, D. The Influence of Nutritional Status at Diagnosis of Childhood B-Cell Acute Lymphoblastic Leukemia on Survival Rates: Data from a Hispanic Cohort. Nutr. Cancer 2022, 74, 889–895.
- Suzuki, D.; Kobayashi, R.; Sano, H.; Hori, D.; Kobayash, K. Sarcopenia after induction therapy in childhood acute lymphoblastic leukemia: Its clinical significance. Int. J. Hematol. 2018, 107, 486–489.
- Tah, P.C.; Nik Shanita, S.; Pohm, B.K. Nutritional status among pediatric cancer patients: A comparison between hematological malignancies and solid tumors. J. Spec. Pediatr. Nurs. 2012, 17, 301–311.
- Yoruk, M.A.; Durakbasa, C.U.; Timur, C.; Sahin, S.S.; Taskin, E.C. Assessment of Nutritional Status and Malnutrition Risk at Diagnosis and Over a 6-Month Treatment Period in Pediatric Oncology Patients with Hematologic Malignancies and Solid Tumors. J. Pediatr. Hematol. Oncol. 2018, 41, e308–e321.
- Viani, K.; Barr, R.D.; Filho, V.O.; Ladas, E.J. Nutritional status at diagnosis among children with cancer referred to a nutritional service in Brazil. Hematol. Transfus. Cell Ther. 2021, 43, 389–395.
- Garófolo, A.; Lopez, F.A.; Petrilli, A.S. High prevalence of malnutrition among patients with solid non-hematological tumors as found by using skinfold and circumference measurements. Sao Paulo Med. J. 2005, 123, 277–281.
- Dos Lemos, P.S.M.; de Oliveira, F.L.C.; Caran, E.M.M. Nutritional Status of Children and Adolescents at Diagnosis of Hematological and Solid Malignancies. Rev. Bras. Hematol. Hemoter. 2014, 36, 420–423.
- Radhakrishnan, V.; Ganesan, P.; Rajendranath, R.; Ganesan, T.S.; Sagar, T.G. Nutritional Profile of Pediatric Cancer Patients at Cancer Institute, Chennai. Indian J. Cancer 2015, 52, 207.
- Joffe, L.; Dwyer, S.; Glade, B.J.L.; Frazier, A.L.; Ladas, E.J. Nutritional status and clinical outcomes in pediatric patients with solid tumors: A systematic review of the literature. Semin. Oncol. 2019, 46, 48–56.
- Goldstein, G.; Shemesh, E.; Frenkel, T.; Jacobson, J.M.; Toren, A. Abnormal body mass index at diagnosis in patients with Ewing sarcoma is associated with inferior tumor necrosis. Pediatr. Blood Cancer 2015, 62, 1892–1896.
- Sharib, J.M.; Cyrus, J.; Horvai, A.; Gray, H.F.K.; Neuhaus, J.; Matthay, K.K.; Goldsby, R.; Marina, N.; DuBois, S.G. Predictors of acute chemotherapy-associated toxicity in patients with Ewing sarcoma. Pediatr. Blood Cancer 2012, 59, 611–616.
- Altaf, S.; Enders, F.; Jeavons, E.; Krailo, M.; Barkauskas, D.A.; Meyers, P.; Arndt, C. High-BMI at diagnosis is associated with inferior survival in patients with osteosarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2013, 60, 2042–2046.
- Hingorani, P.; Seidel, K.; Krailo, M.; Mascarenhas, L.; Meyers, P.; Marina, N.; Conrad, E.U.; Hawkins, D.S. Body mass index (BMI) at diagnosis is associated with surgical wound complications in patients with localized osteosarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2011, 57, 939–942.
- Tenardi, R.D.; Frühwald, M.C.; Jürgens, H.; Hertroijs, D.; Bauer, J. Nutritional status of children and young adults with Ewing sarcoma or osteosarcoma at diagnosis and during multimodality therapy. Pediatr. Blood Cancer 2012, 59, 621–626.
- Burke, M.E.; Lyden, E.R.; Meza, J.L.; Ladas, E.J.; Dasgupta, R.; Wiegner, E.A.; Arndt, C.A. Does body mass index at diagnosis or weight change during therapy predict toxicity or survival in intermediate risk rhabdomyosarcoma? A report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Pediatr, Blood Cancer 2013, 60, 748–753.
- Lifson, L.F.; Hadley, G.P.; Wiles, N.L.; Pillay, K. Nutritional status of children with Wilms’ tumour on admission to a South African hospital and its influence on outcome. Pediatr. Blood Cancer 2017, 64, e26382.
- Fernandez, C.V.; Anderson, J.; Breslow, N.E.; Dome, J.S.; Grundy, P.E.; Perlman, E.J.; Green, D.M. Anthropomorphic measurements and event-free survival in patients with favorable histology Wilms tumor: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2009, 52, 254–258.
- Small, A.G.; Thwe, L.M.; Byrne, J.A.; Lau, L.; Chan, A.; Craig, M.E.; Cowell, C.T.; Garnett, S.P. Neuroblastoma, body mass index, and survival: A retrospective analysis. Medicine 2015, 94, e713.
- Aarnivala, H.; Pokka, T.; Soininen, R.; Möttönen, M.; Harila-Saari, A.; Niinimäki, R. Trends in age- and sex-adjusted body mass index and the prevalence of malnutrition in children with cancer over 42 months after diagnosis: A single-center cohort study. Eur. J. Pediatr. 2020, 179, 91–98.
- Tazi, I.; Hidane, Z.; Zafad, S.; Harif, M.; Benchekroun, S.; Ribeiro, R. Nutritional status at diagnosis of children with malignancies in Casablanca. Pediatr. Blood Cancer 2008, 51, 495–498.
- Joffe, L.; Shen, W.; Shadid, G.; Jin, Z.; Ladas, E.J. Skeletal muscle and adipose tissue changes in the first phase of treatment of pediatric solid tumors. Cancer Med. 2021, 10, 15–22.
- Tsutsumi, R.C.; Speridião, P.G.L. Children with brain tumors: A study of nutritional status on hospital admission. Semin. Ciências Biol. Saúde 2021, 42, 51.
- Musiol, K.; Sobol, G.; Mizia-Malarz, A.; Wos, H. Leptin concentration and nutritional status in the course of treatment in children with brain tumours—Preliminary report. Childs Nerv. Syst. 2014, 30, 131–136.
- Barr, R.D.; Ladas, E.J. The role of nutrition in pediatric oncology. Expert Rev. Anticancer Ther. 2020, 20, 109–116.
- Marcucci, G.; Beltrami, G.; Tamburini, A.; Body, J.J.; Confavreux, C.B.; Hadji, P.; Holzer, G.; Kendler, D.; Napoli, N.; Pierroz, D.D.; et al. Bone health in childhood cancer: Review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann. Oncol. 2019, 30, 908–920.
- Perry, B.; Wang, Y. Appetite regulation and weight control: The role of gut hormones. Nutr. Diabetes 2012, 2, e26.
- Woodward, O.R.M.; Gribble, F.M.; Reimann, F.; Lewis, J.E. Gut peptide regulation of food intake—Evidence for the modulation of hedonic feeding. J. Physiol. 2022, 600, 1053–1078.
- Hariyanto, T.I.; Kurniawan, A. Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment. Cancer Treat. Res. Commun. 2021, 27, 100336.
- Minor, R.K.; Chang, J.W.; de Cabo, R. Hungry for life: How the arcuate nucleus and neuropeptide Y may play a critical role in mediating the benefits of calorie restriction. Mol. Cell. Endocrinol. 2009, 299, 79–88.
- Rios, M. BDNF and the central control of feeding: Accidental bystander or essential player? Trends Neurosci. 2013, 36, 83–90.
- Austin, J.; Marks, D. Hormonal regulators of appetite. Int. J. Pediatr. Endocrinol. 2009, 2009, 141753.
- Camilleri, M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015, 148, 1219–1233.
- Suzuki, K.; Simpson, K.A.; Minnion, J.S.; Shillito, J.C.; Bloom, S.R. The role of gut hormones and the hypothalamus in appetite regulation. Endocr. J. 2010, 57, 359–372.
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261.
- Sovetkina, A.; Nadir, R.; Fung, J.N.M.; Nadjarpour, A.; Beddoe, B. The Physiological Role of Ghrelin in the Regulation of Energy and Glucose Homeostasis. Cureus 2020, 12, e7941.
- Davis, M.P.; Dreicer, R.; Walsh, D.; Lagman, R.; LeGrand, S.B. Appetite and cancer-associated anorexia: A review. J. Clin. Oncol. 2004, 22, 1510–1517.
- Wolf, I.; Sadetzki, S.; Kanety, H.; Kundel, Y.; Pariente, C.; Epstein, N.; Oberman, B.; Catane, R.; Kaufman, B.; Shimon, I. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer 2006, 106, 966–973.
- Kerem, M.; Ferahkose, Z.; Yilmaz, U.T.; Pasaoglu, H.; Ofluoglu, E.; Bedirli, A.; Salman, B.; Sahin, T.T.; Akin, M. Adipokines and ghrelin in gastric cancer cachexia. World J Gastroenterol. 2008, 14, 3633–3641.
- Salehi, M.; Purnell, J.Q. The Role of Glucagon-Like Peptide-1 in Energy Homeostasis. Metab. Syndr. Relat. Disord. 2019, 17, 183–191.
- Price, S.L.; Bloom, S.R. Protein PYY and its role in metabolism. Front. Horm. Res. 2014, 42, 147–154.
- Little, T.J.; Horowitz, M.; Feinle-Bisset, C. Role of cholecystokinin in appetite control and body weight regulation. Obes. Rev. 2005, 6, 297–306.
- Rehfeld, J.F. Cholecystokinin-From Local Gut Hormone to Ubiquitous Messenger. Front. Endocrinol. 2017, 8, 47.
- Czogała, W.; Strojny, W.; Schab, M.; Grabowska, A.; Miklusiak, K.; Kowalczyk, W.; Łazarczyk, A.; Tomasik, P.; Skoczeń, S. FTO and PLAG1Genes Expression and FTO Methylation Predict Changes in Circulating Levels of Adipokines and Gastrointestinal Peptides in Children. Nutrients 2021, 13, 3585.
- Ezeoke, C.C.; Morley, J.E. Pathophysiology of anorexia in the cancer cachexia syndrome. J. Cachexia Sarcopenia Muscle 2015, 6, 287–302.
- Fayh, A.P.T.; de Lima Bezerra, A.D.; Friedman, R. Appetite hormones in children and adolescents with cancer: A systematic review of observational studies. Nutr. Hosp. 2018, 35, 201–210.
- Park, S.H.; Jung, M.H.; Chung, N.G.; Suh, B.K.; Lee, B.C. Serum ghrelin and leptin concentrations in children with cancer: Comparisons with normal children. Korean J. Pediatr. 2007, 50, 90511.
- Argyrou, C.; Hatziagapiou, K.; Theodorakidou, M.; Nikola, O.A.; Vlahopoulos, S.; Lambrou, G.I. The role of adiponectin, LEPTIN, and ghrelin in the progress and prognosis of childhood acute lymphoblastic leukemia. Leuk. Lymphoma 2019, 60, 2158–2169.
- Gomes, C.C.; Silva, C.C.G.D.; Nascimento, P.R.P.D.; Lemos, T.M.A.M.; Marcadenti, A.; Markoski, M.M.; Fayh, A.P.T. Nutritional status and appetite-regulating hormones in early treatment of acute lymphoblastic leukemia among children and adolescents: A cohort study. Sao Paulo Med. J. 2020, 138, 118–125.
- Barbosa-Cortés, L.; Klunder-Klunder, M.; López-Alarcón, M.; Márquez, H.R.; López-Aguilar, E.; Tapia-Marcial, A. Nutritional status and cytokine concentration during chemotherapy in Mexican children: A longitudinal analysis. Nutrition 2019, 57, 46–51.
- Skoczeń, S.; Rej, M.; Kwiecińska, K.; Pietrys, D.; Tomasik, P.J.; Wójcik, M.; Strojny, W.; Dłużniewska, A.; Klimasz, K.; Fijorek, K.; et al. Gastrointestinal peptides in children before and after hematopoietic stem cell transplantation. BMC Cancer 2020, 20, 306.
- Co-Reyes, E.; Li, R.; Huh, W.; Chandra, J. Malnutrition and obesity in pediatric oncology patients: Causes, consequences, and interventions. Pediatr. Blood Cancer 2012, 15, 1160–1167.
- Suzuki, H.; Asakawa, A.; Amitani, H.; Nakamura, N.; Inui, A. Cancer cachexia—Pathophysiology and management. J. Gastroenterol. 2013, 48, 574–594.
- Skipworth, R.J.; Stewart, G.D.; Dejong, C.H.; Preston, T.; Fearon, K.C. Pathophysiology of cancer cachexia: Much more than host-tumour interaction? Clin. Nutr. 2007, 26, 667–676.
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615.
- Watkins, B.A. Diet, endocannabinoids, and health. Nutr. Res. 2019, 70, 32–39.
- Iannotti, F.A.; Di Marzo, V. The gut microbiome, endocannabinoids and metabolic disorders. J. Endocrinol. 2021, 248, R83–R97.
- Rutkowska, M.; Jamonit, J. Involvement of the Cannabinoid System in the Regulation of Food Intake. Adv. Clin. Exp. Med. 2005, 14, 1011–1017.
- Fride, E.; Foox, A.; Rosenberg, E.; Faigenboim, M.; Cohen, V.; Barda, L.; Blau, H.; Mechoulam, R. Milk intake and survival in newborn cannabinoid CB1 receptor knockout mice: Evidence for a “CB3” receptor. Eur. J. Pharmacol. 2003, 461, 27–34.
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949.
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90.
- Horn, H.; Böhme, B.; Dietrich, L.; Koch, M. Endocannabinoids in Body Weight Control. Pharmaceuticals 2018, 11, 55.
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252.
- Meccariello, R.; Santoro, A.; D’Angelo, S.; Morrone, R.; Fasano, S.; Viggiano, A.; Pierantoni, R. The Epigenetics of the Endocannabinoid System. Int. J. Mol. Sci. 2020, 21, 1113.
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833.
- Tarragon, E.; Moreno, J.J. Cannabinoids, Chemical Senses, and Regulation of Feeding Behavior. Chem. Senses 2019, 44, 73–89.
- Brunt, T.M.; Bossong, M.G. The neuropharmacology of cannabinoid receptor ligands in central signaling pathways. Eur. J. Neurosci. 2022, 55, 909–921.
- Schulz, P.; Hryhorowicz, S.; Rychter, A.M.; Zawada, A.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Role Does the Endocannabinoid System Play in the Pathogenesis of Obesity? Nutrients 2021, 13, 373.
- Blankman, J.L.; Cravatt, B.F. Chemical probes of endocannabinoid metabolism. Pharmacol. Rev. 2013, 65, 849–871.
- Borowska, M.; Czarnywojtek, A.; Sawicka-Gutaj, N.; Woliński, K.; Płazińska, M.T.; Mikołajczak, P.; Ruchała, M. The effects of cannabinoids on the endocrine system. Endokrynol. Pol. 2018, 69, 705–719.
- Andradas, C.; Truong, A.; Byrne, J.; Endersby, R. The Role of Cannabinoids as Anticancer Agents in Pediatric Oncology. Cancers 2021, 13, 157.
- Izzo, A.A.; Sharkey, K.A. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol. Ther. 2010, 126, 21–38.
- Ravinet, T.C.; Delgorge, C.; Menet, C.; Arnone, M.; Soubrié, P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 640–648.
- Cardinal, P.; Bellocchio, L.; Clark, S.; Cannich, A.; Klugmann, M.; Lutz, B.; Marsicano, G.; Cota, D. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology 2012, 153, 4136–4143.
- Abdalla, M.M. Central and peripheral control of food intake. Endocr. Regul. 2017, 51, 52–70.
- Gatta-Cherifi, B.; Cota, D. New insights on the role of the endocannabinoid system in the regulation of energy balance. Int. J. Obes. 2016, 40, 210–219.
- Kola, B.; Farkas, I.; Christ-Crain, M.; Wittmann, G.; Lolli, F.; Amin, F.; Harvey-White, J.; Liposits, Z.; Kunos, G.; Grossman, A.B.; et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE 2008, 3, e1797.
- Di Marzo, V.; Goparaju, S.K.; Wang, L.; Liu, J.; Bátkai, S.; Járai, Z.; Fezza, F.; Miura, G.I.; Palmiter, R.D.; Sugiura, T.; et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001, 410, 822–825.
- Komorowski, J.; Stepień, H. The role of the endocannabinoid system in the regulation of endocrine function and in the control of energy balance in humans. Postepy Hig. Med. Dosw. 2007, 61, 99–105.
- Tagliamonte, S.; Laiola, M.; Ferracane, R.; Vitale, M.; Gallo, M.A.; Meslier, V.; Pons, N.; Ercolini, D.; Vitaglione, P. Mediterranean diet consumption affects the endocannabinoid system in overweight and obese subjects: Possible links with gut microbiome, insulin resistance and inflammation. Eur. J. Nutr. 2021, 60, 3703–3716.
- Tagliamonte, S.; Gill, C.I.R.; Pourshahidi, L.K.; Slevin, M.M.; Price, R.K.; Ferracane, R.; Lawther, R.; O’Connor, G.; Vitaglione, P. Endocannabinoids, endocannabinoid-like molecules and their precursors in human small intestinal lumen and plasma: Does diet affect them? Eur. J. Nutr. 2021, 60, 2203–2215.
- Argenziano, M.; Tortora, C.; Bellini, G.; Di Paola, A.; Punzo, F.; Rossi, F. The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases. Int. J. Mol. Sci. 2019, 20, 5875.
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int. J. Oncol. 2017, 51, 369–377.
- Liu, W.M.; Scott, K.A.; Shamash, J.; Joel, S.; Powles, T.B. Enhancing the in vitro cytotoxic activity of Delta9-tetrahydrocannabinol in leukemic cells through a combinatorial approach. Leuk. Lymphoma 2008, 49, 1800–1809.
- Oesch, S.; Walter, D.; Wachtel, M.; Pretre, K.; Salazar, M.; Guzman, M.; Velasco, G.; Schafer, B.W. Cannabinoid receptor 1 is a potential drug target for treatment of translocation-positive rhabdomyosarcoma. Mol. Cancer Ther. 2009, 8, 1838–1845.
- Fisher, T.; Golan, H.; Schiby, G.; PriChen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. 2016, 23, S15–S22.
- Notaro, A.; Sabella, S.; Pellerito, O.; Di Fiore, R.; De Blasio, A.; Vento, R.; Calvaruso, G.; Giuliano, M. Involvement of PAR-4 in cannabinoid-dependent sensitization of osteosarcoma cells to TRAIL-induced apoptosis. Int. J. Biol. Sci. 2014, 10, 466–478.
- Sredni, S.T.; Huang, C.C.; Suzuki, M.; Pundy, T.; Chou, P.; Tomita, T. Spontaneous involution of pediatric low-grade gliomas: High expression of cannabinoid receptor 1 (CNR1) at the time of diagnosis may indicate involvement of the endocannabinoid system. Childs Nerv. Syst. 2016, 32, 2061–2067.
