Vector-borne infectious diseases (e.g., malaria, dengue fever, and yellow fever) result from a parasite transmitted to humans and other animals by blood-feeding arthropods. They are major contributors to the global disease burden, as they account for nearly a fifth of all infectious diseases worldwide. The interaction between vectors and their hosts plays a key role driving vector-borne disease transmission.
- haemosporidian
- mosquitoes
- parasite manipulation hypothesis
- preen oil
- vector attractants
1. Avian Haemosporidians and Their Vectors
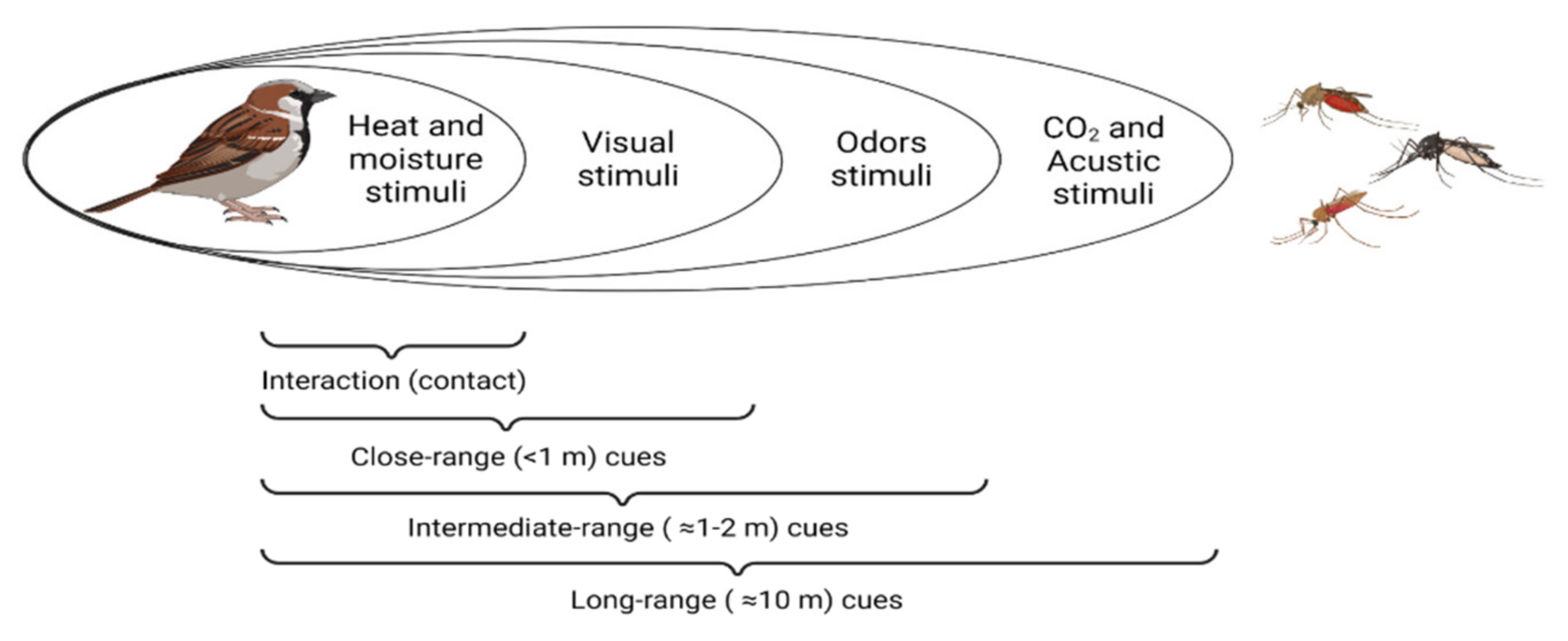
2. Cues Followed by Haemosporidian Vectors to Locate Their Hosts
|
Stimulus |
Host |
Vector |
Effect |
Explanation |
Reference |
|
|---|---|---|---|---|---|---|
|
Visual |
Colour |
49 North American bird species |
Culex pipiens |
+ |
Mosquitoes fed preferably on birds with lighter-coloured plumage. |
[36] |
|
Motion |
Cyanistes caeruleus |
Biting midges |
+ |
Abundance of biting midges was positively associated with parental provisioning effort (increased motion activity). |
[37] |
|
|
Size |
49 North American bird species |
Culex pipiens |
+ |
Mosquitoes fed preferably on birds with longer tarsi. |
[36] |
|
|
Heat and moisture |
Temperature |
Ficedula hypoleuca |
Biting midges |
+ |
Abundance of biting midges increased with temperature inside the bird nests. |
[38] |
|
Temperature |
Parus major |
Culex pipiens |
− |
Birds with a lower body temperature were preferentially chosen by mosquitoes. |
[39] |
|
|
Metabolic rate |
Passer domesticus |
Culex pipiens |
− |
House sparrows with lower metabolic rate suffered more mosquito bites. |
[40] |
|
|
Moisture and temperature |
Cyanistes caerules |
Biting midges and black flies |
0 |
No higher abundance of biting midges and black flies in nests with higher temperature and lower humidity. |
[41] |
|
|
Acoustic |
Bird calls |
Passer, Fringila, Emberiza |
Culex territans |
+ |
60% of female mosquitoes oriented toward the bird songs in phonotaxis experiments. |
[42] |
|
Auditory stimulus |
Upupa epops |
Mosquitoes, blackflies and biting midges |
0 |
Auditory cues of nestling hoopoes did not affect the abundance of vectors. |
[43] |
|
|
Olfactory |
Carbon dioxide (CO2) |
Cyanistes caeruleus |
Biting midges |
+ |
Higher biting midge abundance in nests boxes with CO2 levels higher than in the forest air. |
[44] |
|
Uropygial gland secretions |
Uropygial secretion |
Gavia immer |
Simulium euryadminiculum |
+ |
Black flies were attracted to the odour of the common loon’s uropygial gland. |
[45] |
|
Uropygial secretion |
Gavia immer |
Simulium euryadminiculum |
+ |
Higher attraction of black flies to a combination of ether extract of the uropygial glands and CO2 than to CO2 alone. |
[46] |
|
|
Ether extract |
Gavia immer |
Simulium euryadminiculum |
+ |
Black flies were attracted to ether components of the uropygial gland. |
[47] |
|
|
Cotton swabs coated with uropygial secretions |
Corvus brachyrhynchus |
Culex pipiens, Culex restuans |
+ |
CDC traps baited with uropygial secretions captured more mosquitos than control traps. |
[48] |
|
|
Diol volatile compounds from Natasauropygial gland secretion |
Culex quinquefasciatus Culex tarsalis, Culex nigripalpus, Aedes aegypti |
0 |
Meso-2,3-butanediol, 2,3-butanediol, and 2,3- docosanediol were not attractive to mosquitoes. |
[49] |
||
|
Uropygial secretions |
Columba livia Cyanistes caeruleus |
Biting midges and black flies |
0 |
No differences in the number of vectors captured in CDC traps or nests with this stimulus. |
[50] |
|
|
Uropygial secretions |
Passer domesticus |
Culex pipiens, Aedes caspius |
0 |
Mosquitoes were attracted equally to the ports containing uropygial secretion and to the control in olfactometer assays. |
[51] |
|
|
Uropygial secretions |
Upupa epops |
Biting midges |
− |
Traps baited with uropygial secretion in pine forest significantly captured less biting midges than control traps. |
[43] |
|
|
Haemosporidian infection |
Bird infected with malaria |
Serinus canaria |
Culex pipiens |
+ |
Chronically infected birds attracted more vectors than either uninfected or acutely infected birds. |
[52] |
|
Bird infected with malaria |
Passer domesticus |
Culex pipiens |
+ |
Higher feeding preference of mosquitoes on infected sparrows. |
[53] |
|
|
Bird infected with malaria |
Passer domesticus |
Culex pipiens |
+ |
Mosquitoes were more attracted to the odour of malaria-infected sparrows. |
[54] |
|
|
Bird infected with malaria |
Cyanistes caeruleus |
Biting midges |
− |
Higher abundance of biting midges in the nest attended by medicated birds with reduced parasitaemia. |
[37] |
|
|
Bird infected with malaria |
Parus major |
Culex pipiens |
− |
Plasmodium-infected birds attracted significantly fewer mosquitoes than the uninfected ones. |
[55] |
|
|
Bird infected with malaria |
Corvus monedula Passer domesticus |
Culex pipiens, Aedes caspius |
0 |
Similar biting rates of mosquitoes on malaria infected and uninfected birds. |
[56] |
|
3. The Role of Uropygial Gland Secretion in Bird–Haemosporidian Vector Interactions
4. Do Bird Malaria Parasites Change the Host Attractiveness to Vectors?
4.1. Manipulation of Vector to Increase Parasite Transmission
4.2. Manipulation of Vertebrate Host Attractiveness to Vectors
This entry is adapted from the peer-reviewed paper 10.3390/biology11050726
References
- Carter, R.; Mendis, K.N. Evolutionary and Historical Aspects of the Burden of Malaria. Clin. Microbiol. Rev. 2002, 15, 564.
- WHO. WHO Guidelines for Malaria; World Health Organization: Geneva, Switzerland, 2021.
- Martinsen, E.S.; Perkins, S.L.; Schall, J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol. Phylogenet. Evol. 2008, 47, 261–273.
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0415300975.
- Valkiūnas, G.; Atkinson, C.T. Introduction to Life Cycles, Taxonomy, Distribution, and Basic Research Techniques. In Avian Malaria and Related Parasites in the Tropics; Springer: Cham, Switzerland, 2020; pp. 45–80.
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358.
- Ghaemitalab, V.; Mirshamsi, O.; Valkiūnas, G.; Aliabadian, M. Prevalence and Genetic Diversity of Avian Haemosporidian Parasites in Southern Iran. Pathogens 2021, 10, 645.
- Garcia-Longoria, L.; Muriel, J.; Magallanes, S.; Villa-Galarce, Z.H.; Ricopa, L.; Inga-Díaz, W.G.; Fong, E.; Vecco, D.; Guerra-Saldaña, C.; Salas-Rengifo, T.; et al. Diversity and host assemblage of avian haemosporidians in different terrestrial ecoregions of Peru. Curr. Zool. 2021, 68, 27–40.
- Muriel, J.; Marzal, A.; Magallanes, S.; García-Longoria, L.; Suarez-Rubio, M.; Bates, P.J.J.; Lin, H.H.; Soe, A.N.; Oo, K.S.; Aye, A.A.; et al. Prevalence and diversity of avian haemosporidians may vary with anthropogenic disturbance in tropical habitats in myanmar. Diversity 2021, 13, 111.
- Santiago-Alarcón, D.; Marzal, A. Avian Malaria and Related Parasites in the Tropics. Available online: https://link.springer.com/book/10.1007/978-3-030-51633-8 (accessed on 1 January 2022).
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science 2015, 347, 436–438.
- Martínez-De La Puente, J.; Merino, S.; Tomás, G.; Moreno, J.; Morales, J.; Lobato, E.; García-Fraile, S.; Belda, E.J. The blood parasite Haemoproteus reduces survival in a wild bird: A medication experiment. Biol. Lett. 2010, 6, 663–665.
- Marzal, A.; Balbontín, J.; Reviriego, M.; García-Longoria, L.; Relinque, C.; Hermosell, I.G.; Magallanes, S.; López-Calderón, C.; de Lope, F.; Møller, A.P. A longitudinal study of age-related changes in Haemoproteus infection in a passerine bird. Oikos 2016, 125, 1092–1099.
- Marzal, A.; de Lope, F.; Navarro, C.; Møller, A.P. Malarial parasites decrease reproductive success: An experimental study in a passerine bird. Oecologia 2005, 142, 541–545.
- Merino, S.; Moreno, J.; Jose, J.; Arriero, E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). R. Soc. 2000, 9, 2507–2510.
- Ilgunas, M.; Bukauskaite, D.; Palinauskas, V.; Iezhova, T.; Fragner, K.; Platonova, E.; Weissenböck, H.; Valkiūnas, G. Patterns of Plasmodium homocircumflexum virulence in experimentally infected passerine birds. Malar. J. 2019, 18, 174.
- Marzal, A.; Garcia-Longoria, L. The Role of Malaria Parasites in Invasion Biology. In Avian Malaria and Related Parasites in the Tropics; Springer: Cham, Switzerland, 2020; pp. 487–512.
- Clark, N.J.; Clegg, S.M.; Lima, M.R. A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New insights from molecular data. Int. J. Parasitol. 2014, 44, 329–338.
- Perez-Tris, J.; Hasselquist, D.; Hellgren, O.; Krizanauskiene, A.; Waldenström, J.; Bensch, S. What are malaria parasites? Trends Parasitol. 2005, 21, 209–211.
- Valkiūnas, G.; Anwar, A.M.; Atkinson, C.; Greiner, E.; Paperna, I.; Peirce, M. What distinguishes malaria parasites from other pigmented haemosporidians? Trends Parasitol. 2005, 21, 357–358.
- Perkins, S.L. Malaria’s many mates: Past, present, and future of the systematics of the order haemosporida. J. Parasitol. 2014, 100, 11–25.
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian malaria parasites. Malar. J. 2018, 17, 212.
- Cornet, S.; Nicot, A.; Rivero, A.; Gandon, S. Evolution of Plastic Transmission Strategies in Avian Malaria. PLOS Pathog. 2014, 10, e1004308.
- Ferreira, F.C.; Santiago-Alarcon, D.; Braga, É.M. Diptera Vectors of Avian Haemosporidians: With Emphasis on Tropical Regions. In Avian Malaria and Related Parasites in the Tropics; Springer: Cham, Switzerland, 2020; pp. 185–250.
- Ejiri, H.; Sato, Y.; Sasaki, E.; Sumiyama, D.; Tsuda, Y.; Sawabe, K.; Matsui, S.; Horie, S.; Akatani, K.; Takagi, M.; et al. Detection of avian Plasmodium spp. DNA sequences from mosquitoes captured in Minami Daito Island of Japan. J. Vet. Med. Sci. 2008, 70, 1205–1210.
- Ferreira, F.C.; Rodrigues, R.A.; Sato, Y.; Borges, M.A.Z.; Braga, É.M. Searching for putative avian malaria vectors in a Seasonally Dry Tropical Forest in Brazil. Parasites Vectors 2016, 9, 587.
- Santiago-Alarcon, D.; Palinauskas, V.; Schaefer, H.M. Diptera vectors of avian Haemosporidian parasites: Untangling parasite life cycles and their taxonomy. Biol. Rev. 2012, 87, 928–964.
- Gabaldon, A.; Zerpa, N. Fallisia (Plasmodioides) neotropicalis subgen. nov. sp. nov. from Venezuela. Parasitology 1985, 90, 217–225.
- Takken, W. Push-pull strategies for vector control. Malar. J. 2010, 9, 1.
- Thongsripong, P.; Hyman, J.M.; Kapan, D.D.; Bennett, S.N. Human-Mosquito Contact: A Missing Link in Our Understanding of Mosquito-Borne Disease Transmission Dynamics. Ann. Entomol. Soc. Am. 2021, 114, 397–414.
- Atkinson, C.T.; Van Riper, C., III. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon and Haemoproteus. In Bird-Parasite Interactions: Ecology, Evolution, and Behaviour; Oxford University Press: Oxford, UK, 1991; pp. 19–48. ISBN 0198577389.
- Martínez-de la Puente, J.; Dunn, J.C.; Gangoso, L. Factors Affecting Host Selection by Mosquitoes: Implications for the Transmission of Vector-Borne Pathogens; Frontiers Media SA: Lausanne, Swtizerland, 2021.
- Ganser, C.; Monadjem, A.; McCleery, R.A.; Ndlela, T.; Wisely, S.M. Is it best on the nest? Effects of avian life-history on haemosporidian parasitism. Int. J. Parasitol. Parasites Wildl. 2020, 13, 62–71.
- Yan, J.; Gangoso, L.; Ruiz, S.; Soriguer, R.; Figuerola, J.; Martínez-de la Puente, J. Understanding host utilization by mosquitoes: Determinants, challenges and future directions. Biol. Rev. 2021, 96, 1367–1385.
- Raji, J.I.; DeGennaro, M. Genetic analysis of mosquito detection of humans. Curr. Opin. Insect Sci. 2017, 20, 34–38.
- Yan, J.; Gangoso, L.; Martínez-de la Puente, J.; Soriguer, R.; Figuerola, J. Avian phenotypic traits related to feeding preferences in two Culex mosquitoes. Sci. Nat. 2017, 104, 76.
- Tomás, G.; Merino, S.; Martínez-De La Puente, J.; Moreno, J.; Morales, J.; Lobato, E. Determinants of abundance and effects of blood-sucking flying insects in the nest of a hole-nesting bird. Oecologia 2008, 156, 305–312.
- Martínez-de la Puente, J.; Merino, S.; Lobato, E.; de Aguilar, J.R.; del Cerro, S.; Ruiz-de-Castañeda, R.; Moreno, J. Nest-climatic factors affect the abundance of biting flies and their effects on nestling condition. Acta Oecologica 2010, 36, 543–547.
- Cozzarolo, C.S.; Sironi, N.; Glaizot, O.; Pigeault, R.; Christe, P. Sex-biased parasitism in vector-borne disease: Vector preference? PLoS ONE 2019, 14, e0216360.
- Yan, J.; Broggi, J.; Martínez-De La Puente, J.; Gutiérrez-López, R.; Gangoso, L.; Soriguer, R.; Figuerola, J. Does bird metabolic rate influence mosquito feeding preference? Parasites Vectors 2018, 11, 110.
- Castaño-Vázquez, F.; Martínez, J.; Merino, S.; Lozano, M. Experimental manipulation of temperature reduce ectoparasites in nests of blue tits Cyanistes caeruleus. J. Avian Biol. 2018, 49, e01695.
- Bartlett-Healy, K.; Crans, W.; Gaugler, R. Phonotaxis to Amphibian Vocalizations in Culex territans (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2008, 101, 95–103.
- Tomás, G.; Zamora-Muñoz, C.; Martín-Vivaldi, M.; Barón, M.D.; Ruiz-Castellano, C.; Soler, J.J. Effects of Chemical and Auditory Cues of Hoopoes (Upupa epops) in Repellence and Attraction of Blood-Feeding Flies. Front. Ecol. Evol. 2020, 8, 332.
- Castaño-Vázquez, F.; Merino, S.; Cuezva, S.; Sánchez-Moral, S. Nest Gasses as a Potential Attraction Cue for Biting Flying Insects and Other Ectoparasites of Cavity Nesting Birds. Front. Ecol. Evol. 2020, 8, 258.
- Lowther, J.K.; Wood, D.M. Specificity of a Black Fly, Simulium euryadminiculum Davies, toward its Host, the Common Loon. Can. Entomol. 1964, 96, 911–913.
- Fallis, A.M.; Smith, S.M. Ether extracts from birds and CO2 as attractants for some ornithophilic simuliids. Can. J. Zool. 1964, 42, 723–730.
- Bennett, G.F.; Fallis, A.M.; Campbell, A.G. The response of Simulium (Eusimulium) euryadminiculum Davies (Diptera: Simuliidae) to some olfactory and visual stimuli. Can. J. Zool. 1972, 50, 793–800.
- Russell, C.B.; Hunter, F.F. Attraction of Culex pipiens/restuans (Diptera: Culicidae) mosquitoes to bird uropygial gland odors at two elevations in the Niagara region of Ontario. J. Med. Entomol. 2005, 42, 301–305.
- Allan, S.A.; Bernier, U.R.; Kline, D.L. Laboratory Evaluation of Avian Odors for Mosquito (Diptera: Culicidae) Attraction. J. Med. Entomol. 2006, 43, 225–231.
- Martínez-De La Puente, J.; Rivero-De Aguilar, J.; Del Cerro, S.; Argüello, A.; Merino, S. Do secretions from the uropygial gland of birds attract biting midges and black flies? Parasitol. Res. 2011, 109, 1715–1718.
- Díez-Fernández, A.; Martínez-de la Puente, J.; Gangoso, L.; Ferraguti, M.; Soriguer, R.; Figuerola, J. House sparrow uropygial gland secretions do not attract ornithophilic nor mammophilic mosquitoes. Med. Vet. Entomol. 2020, 34, 225–228.
- Cornet, S.; Nicot, A.; Rivero, A.; Gandon, S. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol. Lett. 2013, 16, 323–329.
- Yan, J.; Martínez-de la Puente, J.; Gangoso, L.; Gutiérrez-López, R.; Soriguer, R.; Figuerola, J. Avian malaria infection intensity influences mosquito feeding patterns. Int. J. Parasitol. 2018, 48, 257–264.
- Díez-Fernández, A.; Martínez-de la Puente, J.; Gangoso, L.; López, P.; Soriguer, R.; Martín, J.; Figuerola, J. Mosquitoes are attracted by the odour of Plasmodium-infected birds. Int. J. Parasitol. 2020, 50, 569–575.
- Lalubin, F.; Bize, P.; van Rooyen, J.; Christe, P.; Glaizot, O. Potential evidence of parasite avoidance in an avian malarial vector. Anim. Behav. 2012, 84, 539–545.
- Gutiérrez-López, R.; Martínez-De La Puente, J.; Gangoso, L.; Soriguer, R.; Figuerola, J. Effects of host sex, body mass and infection by avian Plasmodium on the biting rate of two mosquito species with different feeding preferences. Parasites Vectors 2019, 12, 87.
- Vincze, O.; Vágási, C.I.; Kovács, I.; Galván, I.; Pap, P.L. Sources of variation in uropygial gland size in European birds. Biol. J. Linn. Soc. 2013, 110, 543–563.
- Johnston, D.W. Morphological Atlas of the Avian Uropygial Gland (Zoology Bulletins); British Museum (Natural History): London, UK, 1988.
- Hassanin, A.; Shoeib, M.; Massoud, D. Micro- and macroanatomical features of the uropygial gland of duck (Anas platyrhynchos) and pigeon (Columba livia). Biotech. Histochem. 2021, 96, 213–222.
- Jacob, J.; Ziswiler, V. The uropygial gland. In Avian Biology; Elsevier: Amsterdam, The Netherlands, 1982; pp. 199–324.
- Spearman, R.; Hardy, J. Form and Function in Birds; King, A.S., McLelland, J., Eds.; Academic Press: London, UK, 1985.
- Galván, I.; Møller, A.P. Odor Transmission and Olfaction: The Tuft of the Uropygial Gland and Olfactory Ability in Birds. Condor 2013, 115, 693–699.
- Montalti, D.; Gutiérrez, A.M.; Reboredo, G.; Salibián, A. The chemical composition of the uropygial gland secretion of rock dove Columba livia. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2005, 140, 275–279.
- Salibian, A.; Montalti, D. Physiological and biochemical aspects of the avian uropygial gland. Braz. J. Biol. 2009, 69, 437–446.
- Moreno-Rueda, G. Preen oil and bird fitness: A critical review of the evidence. Biol. Rev. 2017, 92, 2131–2143.
- Sweeney, R.J.; Lovette, I.J.; Harvey, E.L. Evolutionary variation in feather waxes of passerine birds. Auk 2004, 121, 435–445.
- Haribal, M.; Dhondt, A.A.; Rosane, D.; Rodriguez, E. Chemistry of preen gland secretions of passerines: Different pathways to same goal? Why? Chemoecology 2005, 15, 251–260.
- Whittaker, D.J.; Soini, H.A.; Atwell, J.W.; Hollars, C.; Novotny, M.V.; Ketterson, E.D. Songbird chemosignals: Volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav. Ecol. 2010, 21, 608–614.
- Amat, J.A.; Rendón, M.A.; Garrido-Fernández, J.; Garrido, A.; Rendón-Martos, M.; Pérez-Gálvez, A. Greater flamingos Phoenicopterus roseus use uropygial secretions as make-up. Behav. Ecol. Sociobiol. 2011, 65, 665–673.
- Elder, W.H. The oil gland of birds. Wilson Bull. 1954, 66, 6–31.
- Jacob, S.; Sallé, L.; Zinger, L.; Chaine, A.S.; Ducamp, C.; Boutault, L.; Russell, A.F.; Heeb, P. Chemical regulation of body feather microbiota in a wild bird. Mol. Ecol. 2018, 27, 1727–1738.
- Møller, A.P.; Laursen, K. Function of the uropygial gland in eiders (Somateria mollissima). Avian Res. 2019, 10, 24.
- Giraudeau, M.; Czirják, G.Á.; Duval, C.; Bretagnolle, V.; Eraud, C.; McGraw, K.J.; Heeb, P. Effect of restricted preen-gland access on maternal self maintenance and reproductive investment in mallards. PLoS ONE 2010, 5, e13555.
- Zhang, J.X.; Wei, W.; Zhang, J.H.; Yang, W.H. Uropygial gland-secreted alkanols contribute to olfactory sex signals in Budgerigars. Chem. Senses 2010, 35, 375–382.
- Zhang, Y.H.; Du, Y.F.; Zhang, J.X. Uropygial gland volatiles facilitate species recognition between two sympatric sibling bird species. Behav. Ecol. 2013, 24, 1271–1278.
- Krause, E.T.; Brummel, C.; Kohlwey, S.; Baier, M.C.; Müller, C.; Bonadonna, F.; Caspers, B.A. Differences in olfactory species recognition in the females of two Australian songbird species. Behav. Ecol. Sociobiol. 2014, 68, 1819–1827.
- Shawkey, M.D.; Pillai, S.R.; Hill, G.E. Chemical warfare? Effects of uropygial oil on feather-degrading bacteria. J. Avian Biol. 2003, 34, 345–349.
- Reneerkens, J.; Versteegh, M.A.; Schneider, A.M.; Piersma, T.; Burtt, E.H. Seasonally changing preen-wax composition: Red knots’ (Calidris canutus) flexible defense against feather-degrading bacteria? Auk 2008, 125, 285–290.
- Martín-Vivaldi, M.; Peña, A.; Peralta-Sánchez, J.M.; Sánchez, L.; Ananou, S.; Ruiz-Rodríguez, M.; Soler, J.J. Antimicrobial chemicals in hoopoe preen secretions are produced by symbiotic bacteria. Proc. R. Soc. B Biol. Sci. 2010, 277, 123–130.
- Ruiz-Rodríguez, M.; Tomás, G.; Martín-Gálvez, D.; Ruiz-Castellano, C.; Soler, J.J. Bacteria and the evolution of honest signals. The case of ornamental throat feathers in spotless starlings. Funct. Ecol. 2015, 29, 701–709.
- Verea, C.; Vitelli–Flores, J.; Isturiz, T.; Rodríguez–Lemoine, V.; Bosque, C. The effect of uropygial gland secretions of Spectacled Thrushes (Turdus nudigenis) on feather degradation and bacterial growth in vitro. J. Ornithol. 2017, 158, 1035–1043.
- Braun, M.S.; Sporer, F.; Zimmermann, S.; Wink, M. Birds, feather-degrading bacteria and preen glands: The antimicrobial activity of preen gland secretions from turkeys (Meleagris gallopavo) is amplified by keratinase. FEMS Microbiol. Ecol. 2018, 94, fiy117.
- Fülöp, A.; Czirják, G.Á.; Pap, P.L.; Vágási, C.I. Feather-degrading bacteria, uropygial gland size and feather quality in House Sparrows Passer domesticus. Ibis 2016, 158, 362–370.
- Møller, A.P.; Czirjak, G.Ã.; Heeb, P. Feather micro-organisms and uropygial antimicrobial defences in a colonial passerine bird. Funct. Ecol. 2009, 23, 1097–1102.
- Ruiz-Rodriguez, M.; Valdivia, E.; Soler, J.J.; Martin-Vivaldi, M.; Martín-Platero, A.M.; Martínez-Bueno, M. Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation. J. Exp. Biol. 2009, 212, 3621–3626.
- Ruiz-Rodríguez, M.; Martínez-Bueno, M.; Martín-Vivaldi, M.; Valdivia, E.; Soler, J.J. Bacteriocins with a broader antimicrobial spectrum prevail in enterococcal symbionts isolated from the hoopoe’s uropygial gland. FEMS Microbiol. Ecol. 2013, 85, 495–502.
- Thomas, R.H.; Price, E.R.; Seewagen, C.L.; Mackenzie, S.A.; Bernards, M.A.; Guglielmo, C.G. Use of TLC-FID and GC-MS/FID to examine the effects of migratory state, diet and captivity on preen wax composition in White-throated Sparrows Zonotrichia albicollis. Ibis 2010, 152, 782–792.
- Gutiérrez, A.M.; Montalti, D.; Reboredo, G.R.; Salibián, A.; Catalá, A. Lindane distribution and fatty acid profiles of uropygial gland and liver of Columba livia after pesticide treatment. Pestic. Biochem. Physiol. 1998, 59, 137–141.
- López-Rull, I.; Pagán, I.; Macías Garcia, C. Cosmetic enhancement of signal coloration: Experimental evidence in the house finch. Behav. Ecol. 2010, 21, 781–787.
- Møller, A.P.; Laursen, K.; Izaguirre, J.; Marzal, A. Antibacterial and anatomical defenses in an oil contaminated, vulnerable seaduck. Ecol. Evol. 2021, 11, 12520–12528.
- Poulin, R.; Maure, F. Host Manipulation by Parasites: A Look Back Before Moving Forward. Trends Parasitol. 2015, 31, 563–570.
- Heil, M. Host manipulation by parasites: Cases, patterns, and remaining doubts. Front. Ecol. Evol. 2016, 4, 80.
- Cozzarolo, C.S.; Glaizot, O.; Christe, P.; Pigeault, R. Enhanced Attraction of Arthropod Vectors to Infected Vertebrates: A Review of Empirical Evidence. Front. Ecol. Evol. 2020, 8, 296.
- Lefèvre, T.; Lebarbenchon, C.; Gauthier-Clerc, M.; Missé, D.; Poulin, R.; Thomas, F. The ecological significance of manipulative parasites. Trends Ecol. Evol. 2009, 24, 41–48.
- Spence Beaulieu, M.R. The role of parasite manipulation in vector-borne diseases. Evol. Med. Public Health 2019, 2019, 106–107.
- Stanczyk, N.M.; Mescher, M.C.; De Moraes, C.M. Effects of malaria infection on mosquito olfaction and behavior: Extrapolating data to the field. Curr. Opin. Insect Sci. 2017, 20, 7–12.
- Rossignol, P.A.; Ribeiro, J.M.; Spielman, A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am. J. Trop. Med. Hyg. 1984, 33, 17–20.
- Rossignol, P.A.; Ribeiro, J.M.C.; Spielman, A. Increased biting rate and reduced fertility in sporozoite-infected mosquitoes. Am. J. Trop. Med. Hyg. 1986, 35, 277–279.
- Cornet, S.; Nicot, A.; Rivero, A.; Gandon, S. Avian malaria alters the dynamics of blood feeding in Culex pipiens mosquitoes. Malar. J. 2019, 18, 4–9.
- Valkiūnas, G.; Iezhova, T.A. Detrimental Effects of Haemoproteus Infections on the Survival of Biting Midge Culicoides impunctatus (Diptera: Ceratopogonidae). J. Parasitol. 2004, 90, 194–196.
- Gutiérrez-López, R.; Martínez-de la Puente, J.; Gangoso, L.; Yan, J.; Soriguer, R.; Figuerola, J. Experimental reduction of host Plasmodium infection load affects mosquito survival. Sci. Rep. 2019, 9, 8782.
- Takken, W.; Verhulst, N.O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 2013, 58, 433–453.
- Nacher, M. Charming the mosquito: Do malaria symptoms increase the attractiveness of the host for the vector? Med. Hypotheses 2005, 64, 788–791.
- Katsuragawa, T.H.; Gil, L.H.S.; Tada, M.S.; Silva, A.D.A.; Costa, J.D.A.N.; Araújo, M.D.S.; Escobar, A.L.; Pereira Da Silva, L.H. The dynamics of transmission and spatial distribution of malaria in riverside areas of Porto Velho, Rondônia, in the Amazon Region of Brazil. PLoS ONE 2010, 5, e9245.
- Coutinho-Abreu, I.V.; Riffell, J.A.; Akbari, O.S. Human attractive cues and mosquito host-seeking behavior. Trends Parasitol. 2021, 38, 246–264.
- Emami, S.N.; Lindberg, B.G.; Hua, S.; Hill, S.R.; Mozuraitis, R.; Lehmann, P.; Birgersson, G.; Borg-Karlson, A.K.; Ignell, R.; Faye, I. A key malaria metabolite modulates vector blood seeking, feeding, and susceptibility to infection. Science 2017, 355, 1076–1080.
- Robinson, A.; Busula, A.O.; Voets, M.A.; Beshir, K.B.; Caulfield, J.C.; Powers, S.J.; Verhulst, N.O.; Winskill, P.; Muwanguzi, J.; Birkett, M.A.; et al. Plasmodium-associated changes in human odor attract mosquitoes. Proc. Natl. Acad. Sci. USA 2018, 115, E4209–E4218.
- Schaber, C.L.; Katta, N.; Bollinger, L.B.; Mwale, M.; Mlotha-Mitole, R.; Trehan, I.; Raman, B.; Odom John, A.R. Breathprinting Reveals Malaria-Associated Biomarkers and Mosquito Attractants. J. Infect. Dis. 2018, 217, 1553–1560.
- Grieves, L.A.; Kelly, T.R.; Bernards, M.A.; Macdougall-Shackleton, E.A. Malarial infection alters wax ester composition of preen oil in songbirds: Results of an experimental study. Auk 2018, 135, 767–776.
- Díez-Fernández, A.; Martínez-de la Puente, J.; Martín, J.; Gangoso, L.; López, P.; Soriguer, R.; Figuerola, J. Sex and age, but not blood parasite infection nor habitat, affect the composition of the uropygial gland secretions in European blackbirds. J. Avian Biol. 2021, 52.
- Busula, A.O.; Verhulst, N.O.; Bousema, T.; Takken, W.; de Boer, J.G. Mechanisms of Plasmodium-Enhanced Attraction of Mosquito Vectors. Trends Parasitol. 2017, 33, 961–973.
- Penn, D.; Potts, W. How do major histocompatibility complex genes influence odor and mating preferences? Adv. Immunol. 1998, 69, 411–436.
- Lacroix, R.; Mukabana, W.R.; Gouagna, L.C.; Koella, J.C. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005, 3, e298.
- Batista, E.P.A.; Costa, E.F.M.; Silva, A.A. Anopheles darlingi (Diptera: Culicidae) displays increased attractiveness to infected individuals with Plasmodium vivax gametocytes. Parasites Vectors 2014, 7, 251.
- Ferguson, H.M.; Rivero, A.; Read, A.F. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology 2003, 127, 9–19.
- De Moraes, C.M.; Stanczyk, N.M.; Betz, H.S.; Pulido, H.; Sim, D.G.; Read, A.F.; Mescher, M.C. Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl. Acad. Sci. USA 2014, 111, 11079–11084.
- Mazorra-Alonso, M.; Tomás, G.; Soler, J.J. Microbially Mediated Chemical Ecology of Animals: A Review of Its Role in Conspecific Communication, Parasitism and Predation. Biology 2021, 10, 274.
- Videvall, E.; Marzal, A.; Magallanes, S.; Fleischer, R.C.; Espinoza, K.; García-Longoria, L. The uropygial gland microbiome of house sparrows with malaria infection. J. Avian Biol. 2021, 52.
