Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
Colorectal cancer is the third leading cause of cancer-related death, and its incidence is rising in the younger patient population. In the past decade, research has unveiled several processes (underlying tumorigenesis, many of which involve interactions between tumor cells and the surrounding tissue or tumor microenvironment (TME). Interactions between components of the TME are mediated at a sub-microscopic level.
- colorectal cancer
- tumor stroma ratio
- tumor microenvironment
1. Tumor Microenvironment in Colorectal Cancer
Histologic biomarkers focus on morphologic aspects of the tumor and its composition rather than its anatomical location and behavior. The substance of the tumor is comprised not only of neoplastic cells but also surrounding stroma which includes immune cells, fibroblasts, signaling molecules and ECM. These components collectively make up the TME. Recent literature about the TME has shed light on CRC tumorigenesis and the complex interactions between tumor cells and the surrounding stroma [1][2][3].
On routine histologic assessment, pathologists can recognize prognostically valuable aspects of the TME, such as variations in tumor stroma, the presence of tumor budding and host inflammatory response [4]. Survival analyses have demonstrated that these histologic parameters may outperform conventional TNM staging [5][6]. Among these new features, the proportion of tumor stroma relative to tumor cells has been identified as an important determinant of tumor progression, especially in CRC [7].
1.1. Stroma
Stromal cells drive tumor progression via the secretion of soluble factors, modulation of the ECM and stimulation of cell migration [8]. Stromal cells provide a scaffold for tumoral cells to grow, supply survival signals including insulin growth factor and CXCL12 and lay down extracellular elements such as collagen, proteoglycans, glycoproteins and integrins [9]. This ECM deposition creates a protective environment for tumor cells by increasing stromal density and tension, which may prevent the efficacy of anticancer agents such as biologics and chemotherapy [8][9].
1.2. Epithelial Mesenchymal Transition
Through secretion of chemokines and growth factors, the TME enables neoplastic epithelial cells to undergo a process referred to as epithelial mesenchymal transition. Tumor cells then acquire a mesenchymal phenotype leading to invasive potential, enhanced migration and subsequent disease progression [10][11]. Similarly, the malignant cells transform the surrounding environment by changing the composition of the stroma [8].
1.3. Immune Cells
As part of the interaction between the tumor cells and the tumor bed, immune cells are thought to represent the antitumoral host response [12]. T lymphocytes are one of the major type of cells present in tumors [10]. CD8 T lymphocytes exert cytotoxic actions and CD4 T lymphocytes activate natural killer cells as well as antigen presenting cells. Together, these actions of CD8 and CD4 T cells control tumor growth. Macrophages, as part of innate immunity, are mobilized in response to stimuli from TME and activate inflammatory responses through different mechanisms. The prognostic value of immune cells within and adjacent to the tumor has been validated by multiple survival studies and different cell populations have been characterized [13].
1.4. Tumor Budding
Another well-studied component of TME is small groups of tumor cells at the invasive front, defined as tumor budding (TB). TB has been linked to adverse oncologic outcomes in CRC such as decreased survival and an increased risk for lymph node metastasis [14]. As a high-risk feature, TB was recently incorporated into guidelines for locally advanced CRC by the European Society for Medical Oncology (ESMO) [15].
1.5. Carcinoma Percentage
In 2007, Mesker et al. were the first to publish on the association between carcinoma percentage (CP) relative to stroma and CRC progression [16]. The authors compared patients with high CP tumors to those with low CP tumors and reported lower overall survival (OS) and DFS in the low CP group [16]. These findings suggest stroma plays an active role in CRC progression and resulted in the development of a scoring system to calculate the amount of stroma as a ratio [17].
2. Tumor Stroma Ratio
Tumor stroma ratio (TSR) is defined as the percentage of the neoplastic cell component relative to the stroma in tumor tissue [18]. TSR is determined by evaluation of hematoxylin and eosin (H&E) stained tissue sections and is considered a biomarker derived from the TME [19].
2.1. Scoring Protocol
Various methodologies to estimate the TSR have been proposed [16][17][20]. The protocol developed by van Pelt et al. has high prognostic impact and can be easily implemented in daily practice [20]. TSR is assessed on the same slides used to determine the T stage. Therefore, the slide(s) with the deepest invasion is selected for evaluation. Next, the ×2.5 or ×5 objective is used to identify areas with the highest percentage of stroma. These areas are evaluated for adequate microscopic fields, which was determined to be one ×10 field (approximately 2.54–2.80 mm2) containing both tumor cells and stroma. Additionally, tumor clusters need to be located at four sides of the microscopic field and approximately 90 degrees from one another. For example, if tumor cells are identified at the 12:00 position, at minimum there must also be tumor cells at the 3:00, 6:00 and 9:00 positions (Figure 1), respectively. Only adequate fields are used to calculate stromal percentage, which is reported in 10% increments. If one 10× field with greater than 50% stroma is identified, the tumor is deemed stroma-high. If no such field is identified, the tumor is deemed stroma-low (Figure 2) [20]. Previous studies have shown that a cutoff of 50% allows for the maximum discriminative power [16].
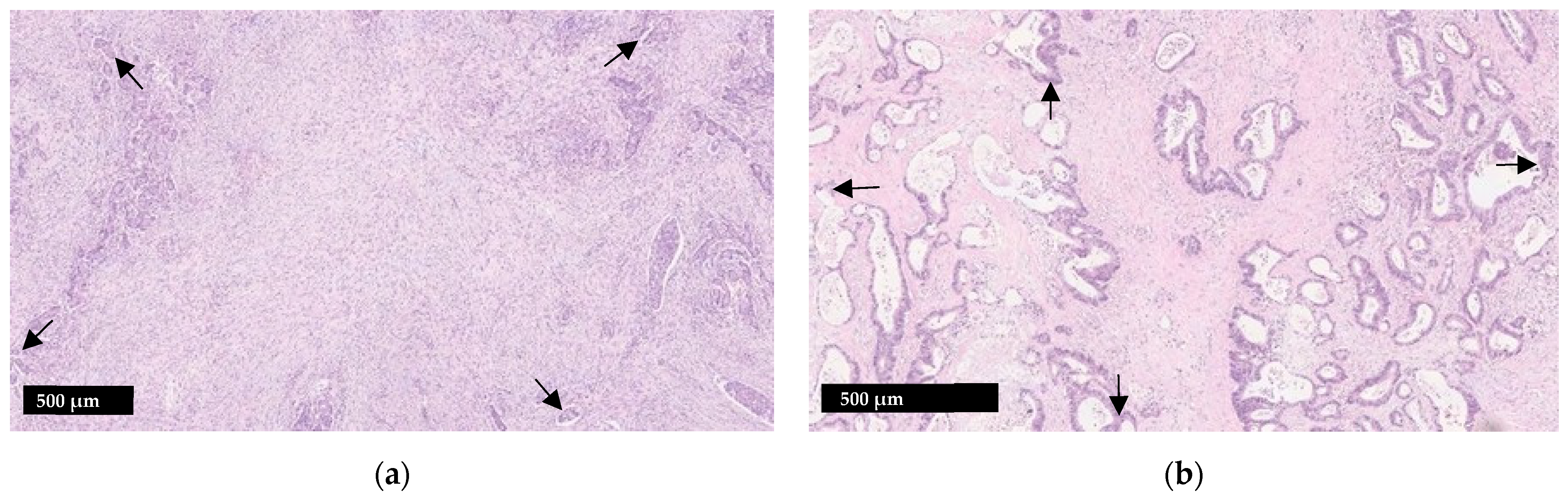
Figure 1. Illustration of the tumor stroma ratio (TSR) (a) Stroma-high tumor; (b) Stroma-low tumor. When assessing adequacy of a visual field, tumor cells should be present at four sides which are roughly 90 degrees from one another (arrows). Smooth muscle, lymphoid follicles and large vessels with thick muscular walls should be disregarded.
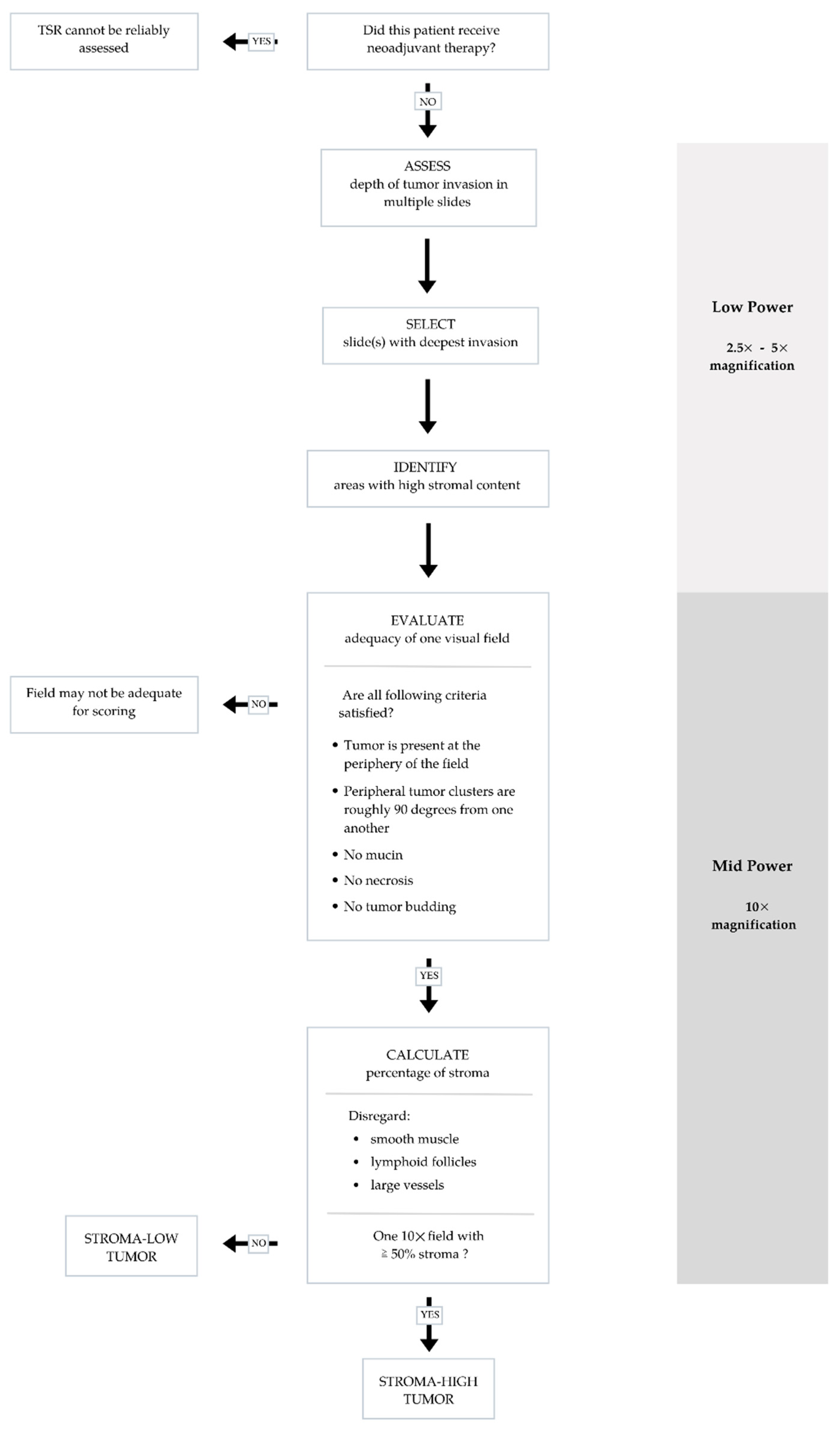
Figure 2. Flow chart summarizing steps to ensure accurate and reproducible evaluation of tumor stromal ratio (TSR) in adequate visual fields.
Other microscopically evident structures such as smooth muscle tissue, lymphoid follicles and large vessels are considered part of the native constituents of the large bowel and should be left out of the microscopic field or disregarded when scoring. Similarly, mucin, necrosis and tumor budding can interfere with scoring and should be avoided as well [17]. TSR scoring is not applicable to specimens from CRC patients who received neoadjuvant therapy as stromal composition may change following neoadjuvant therapy [17].
2.2. Interobserver Variability and Intratumor Heterogeneity
Since TSR is a histologic parameter that can be easily assessed by routine microscopy alone, its reproducibility and feasibility have been scrutinized. Souza et al. reported high interobserver agreement among pathologists scoring TSR in CRC [21]. These results have been validated with several studies reporting moderate to high interobserver agreement in TSR assessment (Cohen’s kappa, range 0.42–0.85) [19][22][23][24].
2.3. Prognostic Value
Tumor associated stroma plays an active role in tumor invasion and metastasis. In a meta-analysis including 4238 patients with solid tumors, the relationship between TSR and prognosis was explored. The authors found that patients with low TSR (stroma-high) were at increased risk of shorter OS and DFS, advanced clinical stage, increased depth of invasion and lymph node metastasis [25]. These findings have been reproduced in subsequent studies which found that stroma-rich tumors had worse outcomes [26][27].
2.4. Tumor Stroma Ratio and Tumor Characteristics
A few studies evaluated associations between TSR and histopathologic tumor characteristics in CRC. Stroma-high CRC tended to have higher T and N stage, resection margin positivity, peritoneal involvement, infiltrative growth at the invasive front and TB, whereas tumor necrosis was more common in stroma-low CRCs (high TSR) [26][28][29]. MSI-H CRCs tended to show high TSR (stroma-low) [29]. There was no difference in gender of the patients, tumor location (colon vs. rectum), tumor differentiation, venous invasion, tumor perforation and local or systemic inflammatory response between the stroma-high and stroma-low groups [26]. Conflicting data exist regarding the associations between age vs. stroma amount [26][30].
2.5. Resistance to Therapy
As is the case with other intrinsic tumor features such as hypoxia, pH and vascular shunting, TSR may contribute to chemoresistance [31]. Hagenaars at al observed that, when compared to their stroma-high counterparts, patients with stroma-low breast tumors were 2.46 times more likely to have a complete response after neoadjuvant therapy [32]. Similar observations have been reported in esophageal carcinoma, suggesting the deleterious effect of stroma in gastrointestinal tumors [33].
In a recent study comparing rectal cancer biopsies, Liang et al. observed a similar trend as patients with high stromal content biopsies were less likely to respond to neoadjuvant treatment. Likewise, the amount of stroma in the pre-treatment biopsy was inversely correlated with the degree of tumor regression [34].
This entry is adapted from the peer-reviewed paper 10.3390/curroncol29050263
References
- Lea, D.; Haland, S.; Hagland, H.R.; Soreide, K. Accuracy of TNM staging in colorectal cancer: A review of current culprits, the modern role of morphology and stepping-stones for improvements in the molecular era. Scand. J. Gastroenterol. 2014, 49, 1153–1163.
- Atiya, H.; Frisbie, L.; Pressimone, C.; Coffman, L. Mesenchymal Stem Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 31–42.
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773.
- Hynes, S.O.; Coleman, H.G.; Kelly, P.J.; Irwin, S.; O’Neill, R.F.; Gray, R.T.; McGready, C.; Dunne, P.D.; McQuaid, S.; James, J.A.; et al. Back to the future: Routine morphological assessment of the tumour microenvironment is prognostic in stage II/III colon cancer in a large population-based study. Histopathology 2017, 71, 12–26.
- Kim, S.; Huh, J.W.; Lee, W.Y.; Yun, S.H.; Kim, H.C.; Cho, Y.B.; Park, Y.A.; Shin, J.K. Prognostic Impact of Lymphatic Invasion, Venous Invasion, Perineural Invasion and Tumor Budding in Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy Followed by Total Mesorectal Excision. Dis. Colon Rectum 2022.
- Qian, X.; Xiao, F.; Chen, Y.Y.; Yuan, J.P.; Liu, X.H.; Wang, L.W.; Xiong, B. Computerized Assessment of the Tumor-stromal Ratio and Proposal of a Novel Nomogram for Predicting Survival in Invasive Breast Cancer. J. Cancer 2021, 12, 3427–3438.
- West, N.P.; Dattani, M.; McShane, P.; Hutchins, G.; Grabsch, J.; Mueller, W.; Treanor, D.; Quirke, P.; Grabsch, H. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br. J. Cancer 2010, 102, 1519–1523.
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15.
- Pietras, K.; Östman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010, 316, 1324–1331.
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729.
- Vincan, E.; Brabletz, T.; Faux, M.C.; Ramsay, R.G. A human three-dimensional cell line model allows the study of dynamic and reversible epithelial-mesenchymal and mesenchymal-epithelial transition that underpins colorectal carcinogenesis. Cells Tissues Organs 2007, 185, 20–28.
- Roxburgh, C.S.; Salmond, J.M.; Horgan, P.G.; Oien, K.A.; McMillan, D.C. Tumour inflammatory infiltrate predicts survival following curative resection for node-negative colorectal cancer. Eur. J. Cancer 2009, 45, 2138–2145.
- Zhao, Y.; Ge, X.; He, J.; Cheng, Y.; Wang, Z.; Wang, J.; Sun, L. The prognostic value of tumor-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 85.
- Koelzer, V.H.; Zlobec, I.; Lugli, A. Tumor budding in colorectal cancer—Ready for diagnostic practice? Hum. Pathol. 2016, 47, 4–19.
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveso, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305.
- Mesker, W.E.; Junggeburt, J.M.C.; Szuhai, K.; de Heer, P.; Morreau, H.; Tanke, H.J.; Tollenaar, R.A.E.M. The Carcinoma–Stromal Ratio of Colon Carcinoma Is an Independent Factor for Survival Compared to Lymph Node Status and Tumor Stage. Cell Oncol. 2007, 29, 175276.
- van Pelt, G.W.; Kjaer-Frifeldt, S.; van Krieken, J.; Al Dieri, R.; Morreau, H.; Tollenaar, R.; Sorensen, F.B.; Mesker, W.E. Scoring the tumor-stroma ratio in colon cancer: Procedure and recommendations. Virchows Arch. 2018, 473, 405–412.
- Pongsuvareeyakul, T.; Khunamornpong, S.; Settakorn, J.; Sukpan, K.; Suprasert, P.; Intaraphet, S.; Siriaunkgul, S. Prognostic evaluation of tumor-stroma ratio in patients with early stage cervical adenocarcinoma treated by surgery. Asian Pac. J. Cancer Prev. 2015, 16, 4363–4368.
- Smit, M.A.; Van Pelt, G.W.; Terpstra, V.; Putter, H.; Tollenaar, R.A.E.M.; Mesker, W.E.; Van Krieken, J.H.J.M. Tumour-stroma ratio outperforms tumour budding as biomarker in colon cancer: A cohort study. Int. J. Colorectal Dis. 2021, 36, 2729–2737.
- van Pelt, G.W.; Sandberg, T.P.; Morreau, H.; Gelderblom, H.; van Krieken, J.; Tollenaar, R.; Mesker, W.E. The tumour-stroma ratio in colon cancer: The biological role and its prognostic impact. Histopathology 2018, 73, 197–206.
- Souza Da Silva, R.M.; Queiroga, E.M.; Paz, A.R.; Neves, F.F.P.; Cunha, K.S.; Dias, E.P. Standardized Assessment of the Tumor-Stroma Ratio in Colorectal Cancer: Interobserver Validation and Reproducibility of a Potential Prognostic Factor. Clin. Pathol. 2021, 14.
- Dang, H.; Van Pelt, G.W.; Haasnoot, K.J.C.; Backes, Y.; Elias, S.G.; Seerden, T.C.J.; Schwartz, M.P.; Spanier, B.W.M.; De Vos Tot Nederveen Cappel, W.H.; Van Bergeijk, J.D.; et al. Tumour-stroma ratio has poor prognostic value in nonpedunculated T1 colorectal cancer: A multicentre case-cohort study. United Eur. Gastroenterol. J. 2021, 9, 478–485.
- Eriksen, A.C.; Andersen, J.B.; Lindebjerg, J.; Depont Christensen, R.; Hansen, T.F.; Kjær-Frifeldt, S.; Sørensen, F.B. Does heterogeneity matter in the estimation of tumour budding and tumour stroma ratio in colon cancer? Diagn. Pathol. 2018, 13, 20.
- Ravensbergen, C.J.; Polack, M.; Roelands, J.; Crobach, S.; Putter, H.; Gelderblom, H.; Tollenaar, R.A.E.M.; Mesker, W.E. Combined Assessment of the Tumor–Stroma Ratio and Tumor Immune Cell Infiltrate for Immune Checkpoint Inhibitor Therapy Response Prediction in Colon Cancer. Cells 2021, 10, 2935.
- Wu, J.; Liang, C.; Chen, M.; Su, W. Association between tumor-stroma ratio and prognosis in solid tumor patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 68954–68965.
- Park, J.H.; Richards, C.H.; McMillan, D.C.; Horgan, P.G.; Roxburgh, C.S.D. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann. Oncol. 2014, 25, 644–651.
- Paulsson, J.; Micke, P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin. Cancer Biol. 2014, 25, 61–68.
- Eriksen, A.C.; Sørensen, F.B.; Lindebjerg, J.; Hager, H.; Depont Christensen, R.; Kjær-Frifeldt, S.; Hansen, T.F. The prognostic value of tumour stroma ratio and tumour budding in stage II colon cancer. A nationwide population-based study. Int. J. Colorectal Dis. 2018, 33, 1115–1124.
- Huijbers, A.; Tollenaar, R.A.E.M.; Pelt, G.W.V.; Zeestraten, E.C.M.; Dutton, S.; McConkey, C.C.; Domingo, E.; Smit, V.T.H.B.M.; Midgley, R.; Warren, B.F.; et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: Validation in the VICTOR trial. Ann. Oncol. 2013, 24, 179–185.
- Zengin, M. Tumour Budding and Tumour Stroma Ratio are Reliable Predictors for Death and Recurrence in Elderly Stage I Colon Cancer Patients. Pathol. Res. Pract. 2019, 215, 152635.
- Tredan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug Resistance and the Solid Tumor Microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454.
- Hagenaars, S.C.; Groot, S.; Cohen, D.; Dekker, T.J.A.; Charehbili, A.; Meershoek-Klein Kranenbarg, E.; Duijm-De Carpentier, M.; Pijl, H.; Putter, H.; Tollenaar, R.A.E.M.; et al. Tumor-stroma ratio is associated with Miller-Payne score and pathological response to neoadjuvant chemotherapy in HER2-negative early breast cancer. Int. J. Cancer 2021, 149, 1181–1188.
- Hale, M.D.; Nankivell, M.; Hutchins, G.G.; Stenning, S.P.; Langley, R.E.; Mueller, W.; West, N.P.; Wright, A.I.; Treanor, D.; Hewitt, L.C.; et al. Biopsy proportion of tumour predicts pathological tumour response and benefit from chemotherapy in resectable oesophageal carcinoma: Results from the UK MRC OE02 trial. Oncotarget 2016, 7, 77565–77575.
- Liang, Y.; Zhu, Y.; Lin, H.; Zhang, S.; Li, S.; Huang, Y.; Liu, C.; Qu, J.; Liang, C.; Zhao, K.; et al. The value of the tumour-stroma ratio for predicting neoadjuvant chemoradiotherapy response in locally advanced rectal cancer: A case control study. BMC Cancer 2021, 21, 729.
This entry is offline, you can click here to edit this entry!
