Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Mitochondria play a key role in the production of metabolic energy in eukaryotic cells. However, apart from energy production, mitochondria also perform several other functions, namely, calcium signaling, cell proliferation, cell cycle regulation, and apoptosis. With growing interest in mitochondria, significant efforts are being made in mitochondria-targeting pharmaceutical interventions, resulting in ‘mitochondrial medicine’ as an emerging area of healthcare research. Mitochondria-targeting nanoparticles (NPs) are now a promising field of drug-delivery systems.
- mitochondrial dysfunction
- ROS
- NP
1. Introduction
Mitochondria play a key role in the production of metabolic energy in eukaryotic cells [1]. However, apart from energy production, mitochondria also perform several other functions, namely, calcium signaling, cell proliferation, cell cycle regulation, and apoptosis (Figure 1) [2]. With growing interest in mitochondria, significant efforts are being made in mitochondria-targeting pharmaceutical interventions, resulting in ‘mitochondrial medicine’ as an emerging area of healthcare research. Mitochondria-targeting nanoparticles (NPs) are now a promising field of drug-delivery systems.
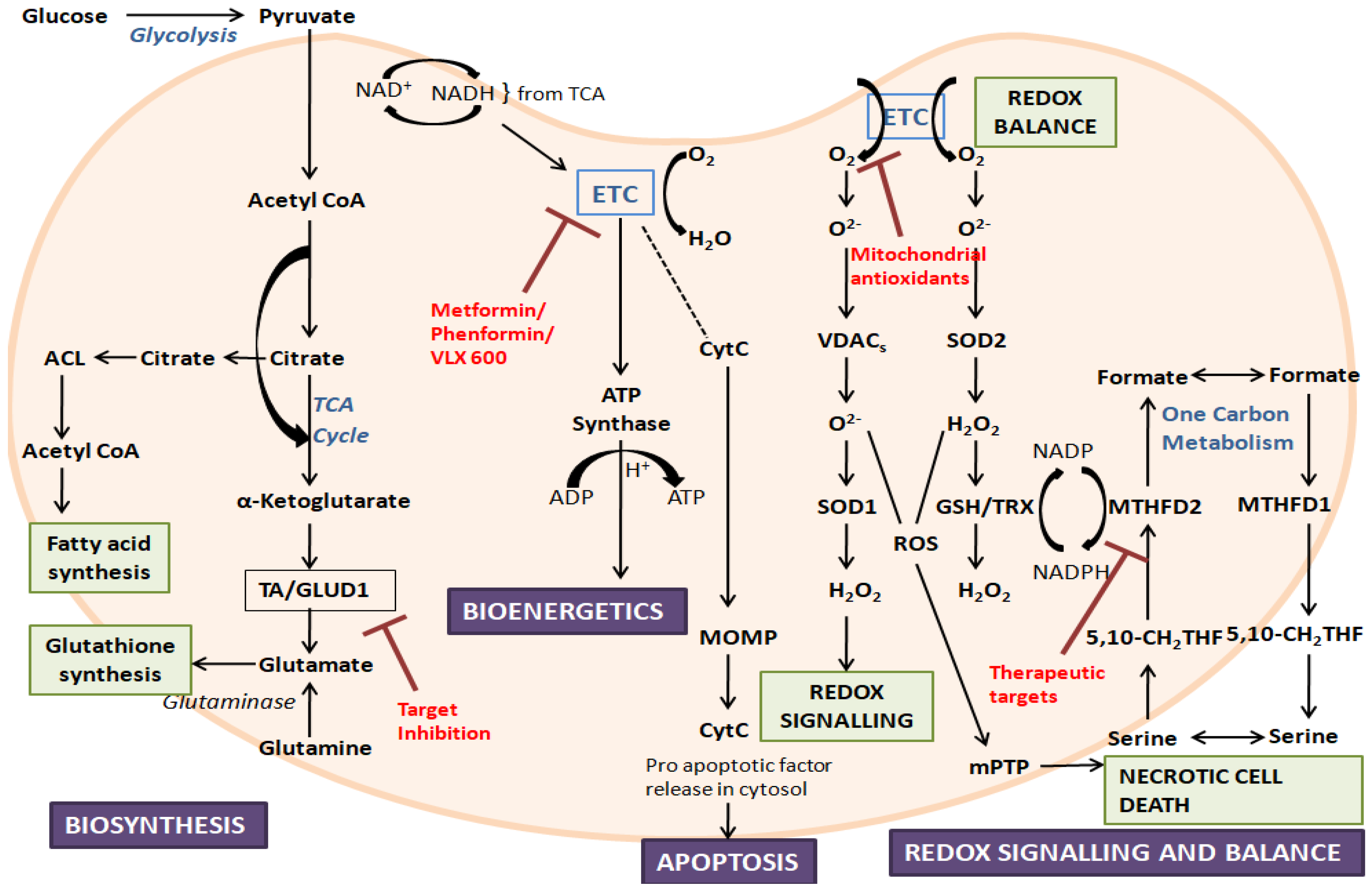
Figure 1. An overview of mitochondrial functions and their dysfunction, along with some target inhibitors: The figure represents the crucial roles played by mitochondria. During disorders, mitochondrial dysfunction can occur due to the alteration of mitochondrial biogenesis, mitochondrial dynamics, ROS production, and mitochondrial-related signaling and apoptosis. ETC, electron transport chain; ACL, ATP citrate lyase; TA, aminotransferase; GLUD1, glutamate dehydrogenase 1; CytC, cytochrome C; TCA, tricarboxylic acid; MOMP, mitochondrial outer membrane permeabilization; ROS, reactive oxygen species; VDAC, voltage-dependent anion channel; SOD, superoxide dismutase; mPTP, mitochondrial permeability transition pore; TRX, thioredoxin; GSH, glutathione; MTHFD, methylene-tetrahydrofolate dehydrogenase; 5,10-CH2-THF, 5,10-methylene-tetrahydrofolate.
Targeting therapeutics to the mitochondria is a challenging task since the mitochondria have four parts, namely, the outer mitochondrial membrane (OMM), the inner mitochondrial membrane (IMM), the intermembrane space (IMS), and the mitochondrial matrix. One of the main hurdles encountered by the molecules in reaching the mitochondrial matrix is the complex membrane network of the mitochondria. Although the therapeutic moieties can pass through the OMM by passive diffusion, phospholipid cardiolipin and a high negative membrane potential cause difficulty for molecules to pass the mitochondrial membranes. The IMM consists of various proteins and ion transporters which play a key role in the electron transport chain and ATP generation [3]. The multiple roles played by the mitochondria make it a target for a variety of therapeutics for the diagnosis and treatment of several diseases.
Low solubility, poor bioavailability, and nonselective biodistribution are some of the present medications’ drawbacks. NPs and conventional chemotherapeutic medicines have recently been combined to produce biocompatible, multifunctional mitochondria-targeted nanoplatforms. Hence, the most promising NP-based techniques for targeting mitochondria in various diseases have been reviewed. This method is now being used to develop targeted medication delivery systems, as well as hybrid nanostructures that can be triggered with light (also known as photodynamic and/or photothermal therapy). The specific delivery of NPs to mitochondria provides an ingenious shortcut to disease treatment that is more selective, precise, and safer. It has the potential to overcome drug resistance while also involving fewer side effects [4].
2. Mitochondrial Dysfunction
Mitochondrial dysfunction can be caused by several factors, such as a defect in the electron transport chain and a reduction in the production of ATP [5]. Reactive oxygen species (ROS) produced by the mitochondria cause the majority of the damage [6]. Mitochondrial dysfunction can occur either because of mutations in the mitochondrial proteins or ROS [7]. When mitochondrial proteins are damaged directly, their affinity for substrates or coenzymes is reduced, and as a result, their activity decreases [8]. Consequently, when a mitochondrion is injured, the cellular requirements for energy-repair processes rise, compromising mitochondrial performance even further [9]. Hyperglycemia causes endothelial cells to produce mitochondrial superoxide, which is a key mediator of diabetes and may lead to consequences such as cardiovascular diseases [10]. Heart failure, atherosclerosis, hypertension, ageing, sepsis, ischemia-reperfusion damage, and hypercholesterolemia are all exacerbated by endothelial superoxide generation [11]. Tumor necrosis factor-alpha (TNF-α), a type of inflammatory mediator, has been linked to mitochondrial dysfunction and enhanced ROS formation in vitro [12]. Mitochondrial dysfunction is a result of metabolic imbalance. Vitamins, minerals, and other metabolites are essential cofactors for the synthesis and activity of mitochondrial enzymes. As a result, micronutrient-deficient diets have been linked to mitochondrial degradation and dementia [13]. Mitochondrial dysfunction is the hallmark of several diseases, such as cancer, neurodegenerative disorders, and cardiovascular disorders [14].
Since the first example was published in 1962, that being a 35-year-old female who suffered from the uncoupling of oxidative phosphorylation (OXPHOS), mitochondrial dysfunction has been linked to practically all pathologic and toxicologic disorders [15].
Diabetes, huntington disease, cancer, alzheimer’s, parkinson’s, schizophrenia, aging, anxiety disorders, cardiovascular diseases, sarcopenia and exercise intolerance are a few acquired conditions associated with mitochondrial dysfunction while, some of the hereditary conditions associated with it include Kearns-Sayre syndrome (KSS), leber hereditary optic neuropathy (LHON), mitochondrial encephalomyopathy, lactic acidosis, and stroke-like syndrome (MELAS), myoclonic epilepsy and ragged-red fibers (MERRF), Leigh syndrome subacute sclerosing encephalopathy, Neuropathy, ataxia, retinitis pigmentosa, and ptosis (NARP), and Myoneurogenic gastrointestinal encephalopathy (MNGIE) [16][17].
2.1. Mitochondrial Biogenesis
Mitochondrial biogenesis refers to the process of producing the new mitochondria in cells [18]. Mitochondrial biogenesis depends on mitochondrial, as well as nuclear, factors. For the formation of new organelles, transcription and translation of both mitochondrial and nuclear genes are required [19]. The expression of genes involved in mitochondrial biogenesis is regulated by nuclear transcription factors. These include the nuclear respiratory factors (NRF1 and NRF2), which play key roles in regulating mitochondrial biogenesis. One of the major regulators of mitochondrial biogenesis is a co-transcriptional regulation factor, PGC-1α, which promotes the expression of Tfam by activating NRF1 and NRF2, which in turn aids in the transcription and replication of mitochondrial DNA (mtDNA) [19].
Several posttranscriptional modifications regulate the activity of PGC-1α, including the phosphorylation of PGC-1α by AMPK and the deacetylation of PGC-1α by silent information regulator two (SIR2) protein 1 (Sirtuin 1, SIRT1) [20].
Drugs interfere with the transcription factor pathways, thus affecting mitochondrial biogenesis [21]. Resveratrol (RSV) promotes mitochondrial biogenesis by activating SIRT1, which causes the acetylation and activation of PGC-1α, or it can also activate AMPK, thus activating PGC-1α independently of SIRT1 [22]. AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) stimulates mitochondrial biogenesis by increasing PGC-1α expression by activating AMPK, and it also mediates mitochondrial apoptosis via the PGC-1α/TFAM pathway in tumor tissues by AMPK phosphorylation [23]. Bezafibrate stimulates mitochondrial biogenesis by activating PPARs and upregulating the PGC-1α coactivator [24].
2.2. Mitochondrial Dynamics
Mitochondrial dynamics involve the processes of fission and fusion [25]. Any dysregulation in these processes leads to either a fragmented network of several pieces of mitochondria or a fused network of several, highly connected mitochondria [26]. Mitochondrial dynamics are affected in several diseases, such as neurodegenerative diseases and cancer [27]. There are several proteins playing key roles in fission and fusion. Proteins involved in mitochondrial fission include dynamin-related/-like protein 1 (Drp1), dynamin 2 (Dnm2), and Fis1. Drp1 forms spiral polymers that surround the mitochondrial membrane and initiate fission by GTP hydrolysis [26][28]. Mitochondrial proteins involved in mitochondrial fusion include mitofusin 1 (Mfn 1), mitofusin 2 (Mfn2), and optic atrophy 1 (OPA1) which involves the fusing together of the outer and the inner mitochondrial membranes [26].
Mdivi-1 was the first Drp1 selective inhibitor [29]. Mdivi-1 shows antiapoptotic activities by inhibiting the mitochondrial membrane permeability. It mitigates cell death in tubular and kidney injury by blocking mitochondrial fission [30]. Another selective inhibitor of Drp2 is P110, which inhibits the activation of Drp1 and the interaction of Drp1 with Fis1, which is required for mitochondrial fission in cultured neuronal cells, thus decreasing mitochondrial fragmentation, ROS production, and neurotoxicity [31].
2.3. Mitochondria-Related Oxidative Stress
ROS are generated as a byproduct due to the regular metabolism of oxygen involving the superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH0) [32][33].
Various autoimmune disorders, cardiovascular disorders, and neurological disorders involve mitochondrial ROS (mtROS) since mitochondria are one of the major sites for the generation of ROS as it activates the RIG-I-like receptors (RLRs), inflammasomes, and mitogen-activated protein kinases (MAPK), which leads to the production of inflammatory cytokines and innate immune responses [34]. MtROS is produced during OXPHOS, and it causes mitochondrial dysfunction by interacting with several components, such as DNA, lipids, and proteins [32].
There are various endogenous antioxidants, such as superoxide dismutase, catalase, and glutathione reductase, which aid in controlling ROS [35]. Coenzyme Q10 (CoQ10)is a lipid antioxidant that aids in preventing the formation of free radicals and any alterations in proteins, lipids, and DNA [36]. Another antioxidant, ferritin, plays a crucial role in sequestering potentially toxic, labile iron and is also involved in ROS-generating Fenton reactions [37][38]. Alpha-lipoic acid is a biological antioxidant that plays a key role in mitochondrial dehydrogenase reactions and recycles vitamin E by interacting with vitamin C and glutathione, thus protecting the membranes [39]. Bilirubin, uric acid, and albumin are other natural antioxidants that scavenge free radicals to prevent oxidative injuries [40][41]. However, it is not easy for the antioxidants to obtain the desired effect because of their restricted distribution in mitochondria. Thus, one of the ways to solve this problem is to attach these antioxidants to triphenylphosphine (TPP+), which can bind with the mitochondrial membrane and result in the severe accumulation of these antioxidants in the mitochondrial matrix [42]. Antioxidants, such as ubiquinone and plastoquinone, have been targeted to the mitochondria in conjugation with TPP+ [43]. Tiron is a mitochondria-localized antioxidant that accumulates within the mitochondria by penetrating the mitochondrial membrane, and it has been found to be effective against UVR-induced oxidative damage [44]. Hemigramicidin-2,2,6,6-tetramethylpiperidine-1-oxyl (Hemigramicidin TEMPO) is a mitochondrion-targeting antioxidant which consists of gramicidin-S and which can be targeted to the mitochondria, independently of the membrane potential and a ROS scavenger TEMPO [45]. Targeting ROS production in mitochondria triggered by SARS-CoV-2 infection may have great potential in drug development against Coronavirus [46].
2.4. Mitochondria Induced Apoptosis
Mitochondria plays a very critical role in the activation of apoptosis in mammalian cells, and it is also involved in other functions, such as energy metabolism, calcium homeostasis, and redox regulation. Thus, mitochondria can be potentially targeted in cancer cells using pharmacological agents as a therapeutic approach [47][48]. One effective way to target and diminish cancer cells is to induce apoptosis. Caspase protease, an enzyme, plays a key role in initiating the apoptosis of a cell. Once the enzyme is active, caspases help in cleaving different types of proteins that ultimately lead to rapid cell death [49]. The most common route of initiating caspase activity is through the mitochondrial pathway. This is achieved via the event of mitochondrial outer-membrane permeabilization (MOMP). Once MOMP occurs, mitochondrial intermembrane space proteins, such as cytochrome-c, are released into the cytosol. These proteins further help in the activation of caspase. Apoptosome formation occurs due to the binding of cytochrome-c in the cytosol with an adaptor molecule called APAF-1. Utilization of death-receptor ligands, such as the TNF-related apoptosis-inducing ligand (TRAIL) to initiate the extrinsic apoptotic pathway can also be a route for inducing mitochondrial apoptosis. Apoptosome helps in the activation of caspases enzymes. The MOMP process is highly effective, which leads to ultimate cell death. It can be highly regulated by the Bcl-2 protein family [50].
2.5. Mitochondria-Related Cell Signaling
Apart from the diverse roles played by mitochondria, they are also involved in the cell-signaling circuitry. They are involved in cell-signaling in two ways: first, by acting as platforms for protein−protein signaling interactions, and second, by regulating levels of intracellular signaling molecules. Mitochondria can affect major signaling mediators, such as ROS, and thus, they are involved in controlling various signaling processes. Mitochondria have several pathophysiological roles because of their involvement in regulating these processes [51]. Mitochondria are also linked to different innate immune-signaling pathways. The most common is the cytosolic RNA-sensing pathway. In this, mitochondria function as an essential platform on which the reactions can take place. During the process of the apoptosis of cells, oxidized mitochondrial DNA activates the NLRP3 inflammasome to activate inflammatory responses [51][52]. Mitochondria also play an important function in the activation and supply of the energy requirements of functioning T cells and macrophages [52].
3. Mitochondria-Targeted, NP-Based Drug Delivery
Mitochondrial dysfunction has been associated with several pathologies that can occur due to alterations in mitochondria-related molecular mechanisms, such as mitochondrial biogenesis, dynamics, mitophagy, and energy metabolism, among other processes.
The steps carried out by the NPs for the transport of the drugs to the mitochondria are shown in Figure 2. The first step involves the intracellular uptake, in which the positively charged NPs bind with the negatively charged phospholipids of the cell membrane. This is followed by endolysosome formation. The endolysosomal membrane then ruptures, causing the release of the contents into the cytoplasm, and the intracellular targeting of the mitochondria takes place [53].
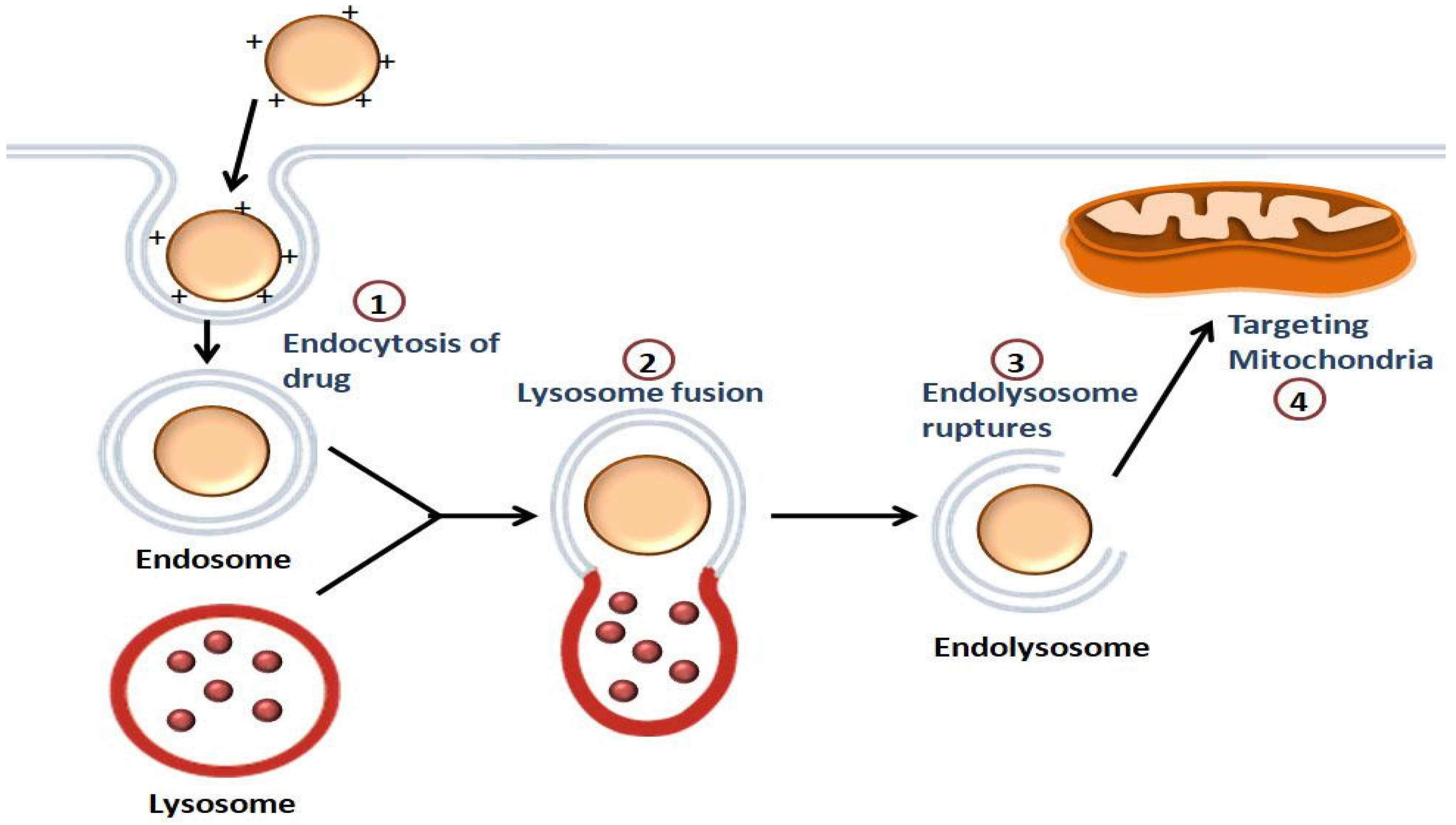
Figure 2. Illustration of the mitochondrial-targeting of drugs by NPs: Endocytosis of the drug occurs, followed by the endolysosome formation. The drug is released into the cytoplasm once the endolysosomal membrane is disrupted, and then the drug is targeted to the mitochondria.
Various barriers within the cell and the mitochondria can be overcome through the design of mitochondria-targeted nanocarriers which have the ability to deliver the drugs selectively to the mitochondria. These nanocarriers aid in the protection of the drug payloads from their elimination and degradation in vivo [7]. Various NP-based drug-delivery systems that can be used for targeting mitochondrial diseases are discussed in here.
3.1. Liposomes
Liposomes are spherical bodies consisting of vesicles made up of phospholipids that contain one or more lipid bilayers and cholesterol. They have an aqueous center that is enclosed within the lipid bilayer [54]. There are around 12 liposome-based drugs available on the market [55]. Liposomes can incorporate both hydrophilic and hydrophobic drugs [54]. Liposomes are used in drug delivery because they are biodegradable, biologically compatible, nontoxic, have the capacity for self-assembly, can carry large drugs, and have several properties which can be altered to control their biological characteristics [7][56].
There are several methods of loading drugs into the liposomes, such as entrapping them in the aqueous region of the liposomes or in the lipophilic bilayers or adsorbing them onto the liposome surface using electrostatic attraction (Figure 3A) [57]. The delivery of therapeutic moieties by mitochondria-targeted, liposome-based drug-delivery systems augments the efficacy of drugs in both in vitro and in vivo models. They are targeted to the mitochondria to encapsulate mitochondria-targeting molecules in lipid bilayers [7].
3.2. Liposome-like Vesicles: DQAsomes
DQAsomes are capable of transporting drugs and DNA to mitochondria [3]. These were the first mitochondria-targeted, vesicular, nanocarrier systems [58]. DeQAlinium (1,1′-Decamethylene bis(4-aminoquinaldinium chloride)) (DQA) consists of two quinaldinium rings linked by ten methylene groups which assemble into liposome-like vesicles called DQAsomes (DeQAlinium-based liposomes) [58]. The mitochondrial membranes of the malignant cells possess a negative electrochemical gradient, in response to which the DQAsomes accumulate in the mitochondria [53].
The plasmid DNA can be delivered into the mitochondria via nonspecific endocytic pathways with the help of DQAsomes. The apoptosis or necrosis is achieved by DQAsomes by hampering the mitochondrial transmembrane potential and disrupting the synthesis of ATP, ROS production, and the activation of MAPK pathways, which ultimately leads to the caspase-dependent apoptotic pathway [53]. Weissig et al., showed that a dicationic amphiphilic compound, DQA, forms liposome-like vesicles called DQAsomes. The plasmid DNA pGL3 firefly luciferase was incorporated into the DQAsomes, and these had transfection efficiencies comparable to those of Lipofectin™. The anticarcinoma activity and selective accumulation of the drug DQA in mitochondria make DQAsome a unique drug-delivery system [59].
3.3. MITO-Porters
A strategy targeting the mitochondrial genome would be effective in delivering the therapeutic agents to the mitochondrial matrix containing the mtDNA pool. MITO-Porter is a liposome-based carrier that delivers macromolecules efficiently to the cytoplasm [60][61], as well as to mitochondria, by membrane fusion [62].
In 2008, Yamada et al. decided to deliver green fluorescence protein (GFP) to rat-liver mitochondria via the membrane fusion mechanism to overcome the limitations of the method by which mitochondrial targeting signal (MTS) peptide is conjugated with exogenous proteins and small linear DNA in order to facilitate their delivery to mitochondria. The plasma membrane is the initial line of defense against intracellular targeting. Yamada et al. coated the MITO-Porter surface with high-density octaarginine (R8), resulting in macropinocytosis instead of clathrin-mediated endocytosis, which allowed particles to enter the cell without being damaged. The MITO-Porter attaches to the mitochondrial membrane via electrostatic interactions after being released from macropinosomes, causing the MITO-Porter and mitochondrion to fuse. The R8-liposomes’ lipid content is crucial since it has to fuse with the mitochondrial membrane. Therefore, the MITO-Porter system is based on the identification of two extremely fusogenic lipid compositions: sphingomyelin (SM) and phosphatidic acid (PA) [62].
In a study by Yasuzaki and colleagues, the aim was to deliver the MITO-Porter carrying the moiety to the mitochondrial matrix in rat-liver mitochondria. Here, membrane-impermeable, red-fluorescent propidium iodide dye used to stain nucleic acids was incorporated into the MITO-Porter liposomes and delivered to the mitochondrial matrix. It was found that the reason behind the localization of these liposomes within the mitochondria was the electrostatic interactions, and the fusogenic lipid components were responsible for the fusion of these liposomes with the mitochondrial membranes [60]. This approach is being researched further for mitochondrial gene therapy [63] and photodynamic cancer therapy [64].
3.4. Micelles
Micelles are amphiphilic molecular systems that are spherical in shape, containing a hydrophobic head group and a hydrophilic tail (Figure 3C). Micelles typically range from 10 to 100 nm in size. These particular types of molecules are formed in aqueous solutions whereby the polar region that is hydrophilic faces the external surface, and the nonpolar region faces the interior part [65][66]. Micelles have garnered attention due to their ability to encapsulate drug substances that are less soluble in water or aqueous environments. Such molecular arrangements are also known as ‘polymeric micelles’, which can deliver drugs of low water-solubility. Micelles have been utilized for mitochondrial-targeted cancer therapies. This drug-delivery mechanism also aids in improving the bioavailability of the drug. The bioavailability of micelles containing drugs can be enhanced by adding external surfactants [66].
3.5. Polymeric NPs
Polymeric NPs are biodegradable and biocompatible and can be targeted to mitochondria for drug delivery because they are easy to manufacture; thus, surface modifications can be made easily and are tunable to drug-release profiles [7]. Polymeric NPs have the properties of higher stability and a more controlled payload release, which are not found in liposomes [3].
Various polymers, such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), and polycaprolactone(PCL), can be used for drug delivery and can be turned into NPs that can incorporate both water-soluble and -insoluble payloads via engineering polyethylene glycol(PEG) to hydrophobic blocks with the help of processes such as emulsification−solvent evaporation or nanoprecipitation. Hydrophobic blocks aid in augmenting stability, whereas PEG helps in increasing the residence time in vivo [7][67].
3.6. Dendrimers
Dendrimers are macromolecules consisting of a central core and multiple branches. These branches have various ligands attached to them on their peripheries. With increased branching, generation numbers, such as G1, G2, G3, G4, and so on, continue to be associated with them (Figure 3B). There are various types of dendrimers, such as peptide dendrimers, liquid crystalline dendrimers, tecto dendrimers, chiral dendrimers, glycodendrimers, polyamidoamine (PAMAM) dendrimers, PAMAM organosilicon dendrimers (PAMAMOS), etc. [68]. Dendrimers are potential nanocarriers for various therapeutic drugs. They are known to show various pharmacokinetic properties, one of which is transdermal drug delivery. It overcomes the side effects of orally given NSAIDs. Several projects are being conducted to use dendrimers in gene therapy as well, the main aim of which is to use dendrimers as potent gene-delivery systems to the cell without causing any harm to the DNA [69]. Dendrimers, when conjugated with lipophilic cations, such as rhodamine or TPP, can deliver drugs to mitochondria via exhibiting endosomal escape properties [3].
Biswas et al., designed the mitochondria-targeted PAMAM dendrimer (G(5)-D). It was prepared in conjunction with TPP on its surface. It was found that these NPs were taken up by the cells efficiently and showed good mitochondria-targeting activity [70]. PAMAM dendrimers, along with TPP, are widely used in targeting mitochondria for drug delivery. In addition, these dendrimers have been proven non-toxic during the process of transfection [3].
3.7. Metal NPs
Metal NPs, such as silver and gold, possess properties such as SPR (surface plasmon resonance) which is not found in liposomes, dendrimers, and micelles. They are also biocompatible and versatile in terms of surface functionalization. Gold NPs (AuNPs) can be conjugated to drugs by ionic or covalent bonds and physical absorption and act as a drug-delivery system, whereas studies have also used silver NPs for the release of ornidazole, which has an in vitro release of 98.5% [71]. Furthermore, gold, silver, and titanium dioxide have been used significantly in recent years as therapeutic tools, and they can be designed to produce very small-sized (3–30 nm), homogenous NPs [7]. Metal NPs are used as potential therapeutics for various infectious diseases because, apart from having good physicochemical properties and surface charges, they also have the ability to conjugate with drugs, antibodies, and proteins, which provides them protection against the host’s immune system, thus increasing their circulation time in the blood [72].
AuNPs have been used as core components while preparing NPs because of their bioinertness, ease of synthesis, and characterization [73]. AuNPs, along with turbo-green fluorescent protein (TGFP) conjugate, have shown an apoptotic effect on breast cancer cells by partially rupturing their mitochondria, and hence, such NPs are helpful in photochemical therapy for cancer [74].
Iron NPs have been used to trigger autophagy in cancerous cells by targeting their mitochondrial DNA [75]. Silver NPs (AgNPs) have also shown potential as nanocarriers for the treatment of various diseases. They have unique antimicrobial, antiviral, and antibacterial properties. Along with this, AgNPs, with or without conjugates, have been seen as potent drug carriers for treating cancer because of their antitumor properties [76].
3.8. Quantum Dots
Quantum dots (QDs) are also known as ‘nanoscale semiconductor crystals’, and they display luminescence. They possess a metalloid crystalline core. It can be used in fluorescence technology, which depends on factors, such as composition and size. QDs in general can range from nanometers to microns. For better fluorescence, the core of QDs can be composed using different materials, such as cadmium–selenium (CdSe), indium–phosphate (InP), etc [77]. QDs can be manufactured using a nanofabrication technology in which properties, such as size, shape, and molecular interactions, can be defined as per requirements [78]. Since QDs are mostly categorized as NPs, they can be advantageous as efficient drug-delivery molecules. Due to their small size, QDs have a larger surface area which can be modified according to biological conditions to lower the aggressive immune response. Moreover, QDs also provide good pharmacokinetic properties [79]. QDs can be coated with biocompatible materials and polymeric materials to enhance solubility and bioactivity once present in vivo. Materials, such as polyhedral oligomeric silsesquioxane-polycarbonateurethane (POSS-PCU) [80] and PEG, can be used [79]. However, metalloid cores can be toxic for human usage. They can be lethal or carcinogenic [81]. Researching biocompatible materials for quantum dots is the current need.
QDs can be utilized in targeted delivery applications. They are usually conjugated with ligands that can be recognized by biological immune systems, such as antibodies, DNA, biotin, streptavidin, and peptides [82]. There are two methods for utilizing QDs as drug-delivery vehicles. First, the drug molecule can be attached to the surface of the QDs. The drugs can be transported to a specific site and can be released when they experience biological phenomena, such as the presence of enzymes. Second, drugs can be encapsulated in a QD−NP system. The entire NP is then transported for site-specific actions. They can be subsequently exposed to conditions where the NP’s outer covering may become degraded or diffused [83]. This was first demonstrated by Bagalkot et al. when they formulated the “QD−Apt(DOX)” complex (QD−aptamer(Apt)−doxorubicin (DOX) as a targeted treatment material for cancer imaging, therapy, and sensing using doxorubicin (DOX),which is an anticancer drug [84].
Carbon QDs or carbon dots are NPs that are fluorescent in nature. They possess efficient optical properties, are photostable in nature, and are highly biocompatible in nature. Due to these attributes, they are usually used in different processes, such as bioimaging, biosensing, and cancer therapy. They also have various functional groups on their cell surfaces which enable the modification of specific functional groups to enhance the process of target-specific drug delivery. Carbon dots, when conjugated with Rose Bengal (RB), a common photosensitizer, form a complex that aids in the cellular uptake of the carbon dot for efficient, mitochondrial-based, photodynamic therapy (PDT) (Figure 3D). Hua et al. demonstrated the production of an efficient carbon dot that can be used for mitochondrial-targeted therapy. They prepared carbon dots via the hydrothermal treatment of a mixture of chitosan, ethylenediamine, and mercaptosuccinic acid. It was also observed and indicated that the cellular uptake of carbon dots is a temperature-dependent process. The temperature at which cells are cultured with carbon dots is an essential factor [85].
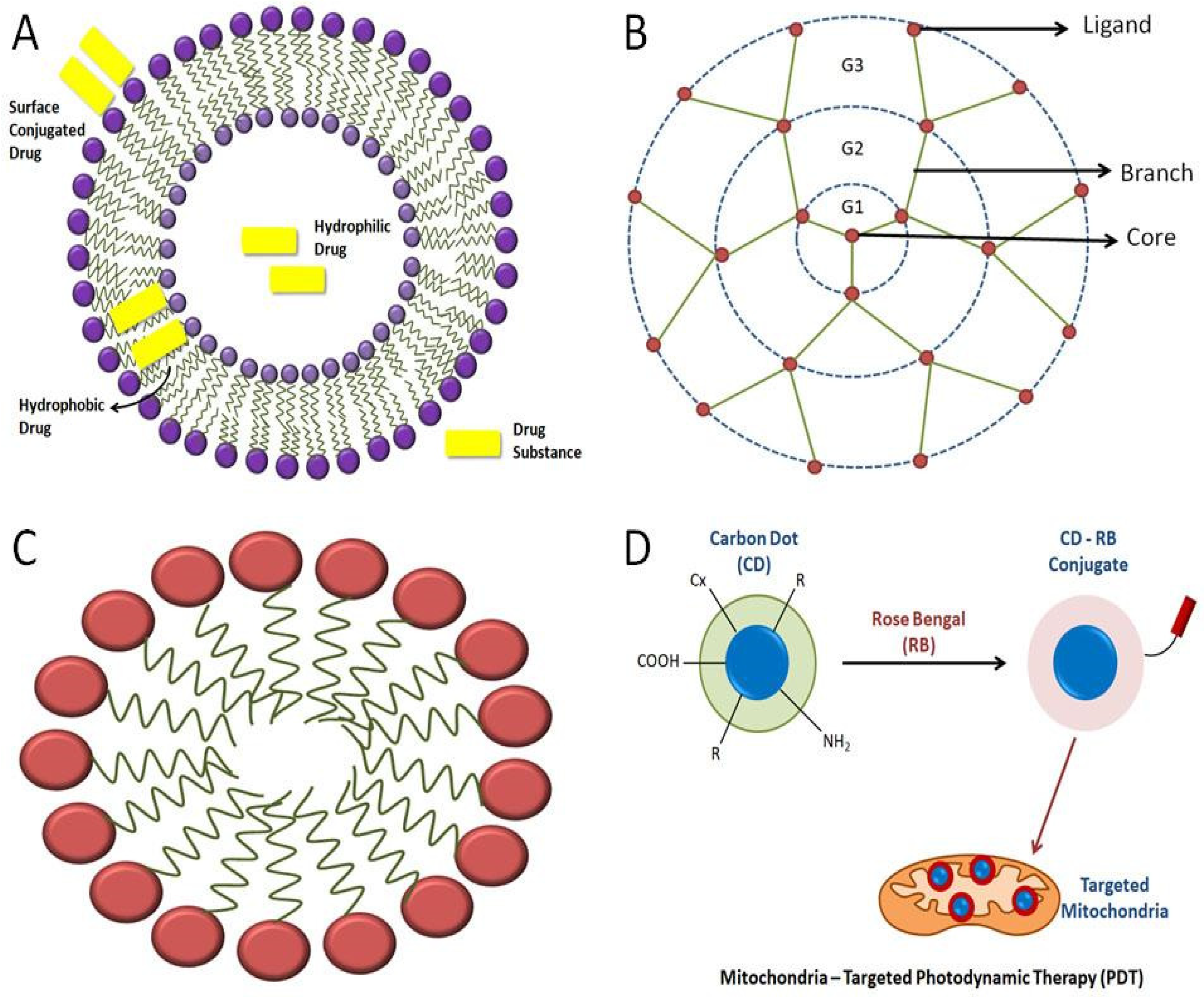
Figure 3. Representation of various kinds of mitochondria-targeting NPs: (A)various strategies of loading drugs into the liposome; (B) basic structure of a dendrimer; (C) general structure of a micelle; (D) carbon quantum dots used in mitochondrial-targeted therapy using Rose Bengal to bring changes in the conjugated carbon-dot molecule, where “R” is a modifiable functional group on carbon quantum dots’ surface
This entry is adapted from the peer-reviewed paper 10.3390/life12050657
References
- Javadov, S.; Kuznetsov, A.V. Mitochondria: The cell powerhouse and nexus of stress. Front. Physiol. 2013, 4, 207.
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723.
- Pathak, R.K.; Kolishetti, N.; Dhar, S. Targeted nanoparticles in mitochondrial medicine. WIREs Nanomed. Nanobiotechnol. 2015, 7, 315–329.
- Tabish, T.A.; Hamblin, M.R. Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer. Biomater. Biosyst. 2021, 3, 100023.
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr. Med. 2014, 13, 35–43.
- Duchen, M.R. Mitochondria in health and disease: Perspectives on a new mitochondrial biology. Mol. Asp. Med. 2004, 25, 365–451.
- Wongrakpanich, A.; Geary, S.M.; Joiner, M.-L.A.; Anderson, M.E.; Salem, A.K. Mitochondria-targeting particles. Nanomedicine 2014, 9, 2531–2543.
- Liu, J.; Killilea, D.W.; Ames, B.N. Age-associated mitochondrial oxidative decay: Improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl- L-carnitine and/or R-α-lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 1876–1881.
- Aw, T.Y.; Jones, D.P. Nutrient Supply and Mitochondrial Function. Annu. Rev. Nutr. 1989, 9, 229–251.
- Green, K.; Brand, M.D.; Murphy, M.P. Prevention of Mitochondrial Oxidative Damage as a Therapeutic Strategy in Diabetes. Diabetes 2004, 53, S110–S118.
- Li, J.-M.; Shah, A.M. Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R1014–R1030.
- Moe, G.W.; Marín-García, J.; König, A.; Goldenthal, M.; Lu, X.; Feng, Q. In Vivo TNF-α inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am. J. Physiol. Circ. Physiol. 2004, 287, H1813–H1820.
- Ames, B.N. Delaying the Mitochondrial Decay of Aging. Ann. N. Y. Acad. Sci. 2004, 1019, 406–411.
- Moos, W.H.; Faller, D.V.; Glavas, I.P.; Harpp, D.N.; Kamperi, N.; Kanara, I.; Kodukula, K.; Mavrakis, A.N.; Pernokas, J.; Pernokas, M.; et al. Pathogenic mitochondrial dysfunction and metabolic abnormalities. Biochem. Pharmacol. 2021, 193, 114809.
- Luft, R.; Ikkos, D.; Palmieri, G.; Ernster, L.; Afzelius, B. A Case of Severe Hypermetabolism of Nonthyroid Origin with a Defect in the Maintenance of Mitochondrial Respiratory Control: A Correlated Clinical, Biochemical, and Morphological Study. J. Clin. Investig. 1962, 41, 1776–1804.
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92.
- Cohen, B.H.; Gold, D.R. Mitochondrial cytopathy in adults: What we know so far. Clevel. Clin. J. Med. 2001, 68, 625–626.
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84.
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2016, 284, 183–195.
- Komen, J.C.; Thorburn, D.R. Turn up the power-pharmacological activation of mitochondrial biogenesis in mouse models. J. Cereb. Blood Flow Metab. 2014, 171, 1818–1836.
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886.
- Ungvari, Z.; Sonntag, W.; de Cabo, R.; Baur, J.; Csiszar, A. Mitochondrial Protection by Resveratrol. Exerc. Sport Sci. Rev. 2011, 39, 128–132.
- Morishita, M.; Kawamoto, T.; Hara, H.; Onishi, Y.; Ueha, T.; Minoda, M.; Katayama, E.; Takemori, T.; Fukase, N.; Kurosaka, M.; et al. AICAR induces mitochondrial apoptosis in human osteosarcoma cells through an AMPK-dependent pathway. Int. J. Oncol. 2016, 50, 23–30.
- Augustyniak, J.; Lenart, J.; Gaj, P.; Kolanowska, M.; Jazdzewski, K.; Stepien, P.P.; Buzanska, L. Bezafibrate Upregulates Mitochondrial Biogenesis and Influence Neural Differentiation of Human-Induced Pluripotent Stem Cells. Mol. Neurobiol. 2018, 56, 4346–4363.
- Ren, L.; Chen, X.; Chen, X.; Li, J.; Cheng, B.; Xia, J. Mitochondrial Dynamics: Fission and Fusion in Fate Determination of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2020, 8, 580070.
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360.
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259.
- Ingerman, E.; Perkins, E.; Marino, M.; Mears, J.; McCaffery, J.M.; Hinshaw, J.E.; Nunnari, J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J. Cell Biol. 2005, 170, 1021–1027.
- Cassidy-Stone, A.; Chipuk, J.E.; Ingerman, E.; Song, C.; Yoo, C.; Kuwana, T.; Kurth, M.J.; Shaw, J.; Hinshaw, J.E.; Green, D.; et al. Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization. Dev. Cell 2008, 14, 193–204.
- Brooks, C.; Wei, Q.; Cho, S.-G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285.
- Qi, X.; Qvit, N.; Su, Y.-C.; Mochly-Rosen, D. A Novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J. Cell Sci. 2013, 126, 789–802.
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077.
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462.
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.-F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19.
- Aguilar, T.A.F.; Navarro, B.C.H.; Pérez, J.A.M. Endogenous Antioxidants: A Review of their Role in Oxidative Stress. Master Regul. Oxid. Stress-Transcr. Factor Nrf2 2016, 3–20.
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467.
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671.
- Salatino, A.; Aversa, I.; Battaglia, A.M.; Sacco, A.; Di Vito, A.; Santamaria, G.; Chirillo, R.; Veltri, P.; Tradigo, G.; Di Cello, A.; et al. H-Ferritin Affects Cisplatin-Induced Cytotoxicity in Ovarian Cancer Cells through the Modulation of ROS. Oxid. Med. Cell. Longev. 2019, 2019, 3461251.
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250.
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996, 2, 161–171.
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science 1987, 235, 1043–1046.
- Wang, J.; Chen, G.-J. Mitochondria as a therapeutic target in Alzheimer’s disease. Genes Dis. 2016, 3, 220–227.
- Broome, S.C.; Woodhead, J.S.T.; Merry, T.L. Mitochondria-Targeted Antioxidants and Skeletal Muscle Function. Antioxidants 2018, 7, 107.
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771.
- Sims, C.R.; MacMillan-Crow, L.A.; Mayeux, P.R. Targeting mitochondrial oxidants may facilitate recovery of renal function during infant sepsis. Clin. Pharmacol. Ther. 2014, 96, 662–664.
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-Da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e5.
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118.
- Wen, S.; Zhu, D.; Huang, P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med. Chem. 2013, 5, 53–67.
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241.
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962.
- Tait, S.; Green, D.R. Mitochondria and cell signalling. J. Cell Sci. 2012, 125 Pt 4, 807–815.
- Zhao, L.; Sumberaz, P. Mitochondrial DNA Damage: Prevalence, Biological Consequence, and Emerging Pathways. Chem. Res. Toxicol. 2020, 33, 2491–2502.
- Bae, Y.; Jung, M.K.; Lee, S.; Song, S.J.; Mun, J.Y.; Green, E.S.; Han, J.; Ko, K.S.; Choi, J.S. Dequalinium-based functional nanosomes show increased mitochondria targeting and anticancer effect. Eur. J. Pharm. Biopharm. 2018, 124, 104–115.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60.
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286.
- Gregoriadis, G.; Perrie, Y. Liposomes. Encyclopedia of Life Sciences; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010.
- Weissig, V. DQAsomes as the Prototype of Mitochondria-Targeted Pharmaceutical Nanocarriers: Preparation, Characterization, and Use. In Mitochondrial Medicine; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1265, pp. 1–11.
- Weissig, V.; Lasch, J.; Erdos, G.; Meyer, H.W.; Rowe, T.C.; Hughes, J. DQAsomes: A Novel Potential Drug and Gene Delivery System Made from Dequalinium. Pharm. Res. 1998, 15, 334–337.
- Yasuzaki, Y.; Yamada, Y.; Harashima, H. Mitochondrial matrix delivery using MITO-Porter, a liposome-based carrier that specifies fusion with mitochondrial membranes. Biochem. Biophys. Res. Commun. 2010, 397, 181–186.
- Yamada, Y.; Furukawa, R.; Yasuzaki, Y.; Harashima, H. Dual Function MITO-Porter, a Nano Carrier Integrating Both Efficient Cytoplasmic Delivery and Mitochondrial Macromolecule Delivery. Mol. Ther. 2011, 19, 1449–1456.
- Yamada, Y.; Akita, H.; Kamiya, H.; Kogure, K.; Yamamoto, T.; Shinohara, Y.; Yamashita, K.; Kobayashi, H.; Kikuchi, H.; Harashima, H. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 423–432.
- Kawamura, E.; Maruyama, M.; Abe, J.; Sudo, A.; Takeda, A.; Takada, S.; Yokota, T.; Kinugawa, S.; Harashima, H.; Yamada, Y. Validation of Gene Therapy for Mutant Mitochondria by Delivering Mitochondrial RNA Using a MITO-Porter. Mol. Ther.-Nucleic Acids 2020, 20, 687–698.
- Munechika, R.; Biju, V.; Takano, Y.; Harashima, H.; Yamada, Y. Satrialdi Correction: The optimization of cancer photodynamic therapy by utilization of a pi-extended porphyrin-type photosensitizer in combination with MITO-Porter. Chem. Commun. 2020, 56, 6153.
- Husseini, G.A.; Pitt, W.G. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152.
- Zhang, Y.; Huang, Y.; Li, S. Polymeric Micelles: Nanocarriers for Cancer-Targeted Drug Delivery. AAPS Pharm. Sci. Tech. 2014, 15, 862–871.
- Wang, Z.; Guo, W.; Kuang, X.; Hou, S.; Liu, H. Nanopreparations for mitochondria targeting drug delivery system: Current strategies and future prospective. Asian J. Pharm. Sci. 2017, 12, 498–508.
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196.
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247.
- Biswas, S.; Dodwadkar, N.S.; Piroyan, A.; Torchilin, V.P. Surface conjugation of triphenylphosphonium to target poly (amidoamine) dendrimers to mitochondria. Biomaterials 2012, 33, 4773–4782.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Aderibigbe, B.A. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules 2017, 22, 1370.
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294.
- Mkandawire, M.M.; Lakatos, M.; Springer, A.; Clemens, A.; Appelhans, D.; Krause-Buchholz, U.; Pompe, W.; Rödel, G. Induction of apoptosis in human cancer cells by targeting mitochondria with gold nanoparticles. Nanoscale 2015, 7, 10634–10640.
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Giampieri, F.; Battino, M.; Bettmer, J.; Montes-Bayón, M.; Llopis, J.; Sánchez-González, C. Ultra-Small Iron Nanoparticles Target Mitochondria Inducing Autophagy, Acting on Mitochondrial DNA and Reducing Respiration. Pharmaceutics 2021, 13, 90.
- Pudlarz, A.; Szemraj, J. Nanoparticles as carriers of proteins, peptides and other therapeutic molecules. Open Life Sci. 2018, 13, 285–298.
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732.
- Kouwenhoven, L.; Marcus, C. Quantum dots. Phys. World 1998, 11, 35–40.
- Ghaderi, S.; Ramesh, B.; Seifalian, A.M. Fluorescence nanoparticles “quantum dot” as drug delivery system and their toxicity: A review. J. Drug Target. 2010, 19, 475–486.
- Kidane, A.G.; Burriesci, G.; Edirisinghe, M.; Ghanbari, H.; Bonhoeffer, P.; Seifalian, A.M. A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater. 2009, 5, 2409–2417.
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172.
- Bharali, D.J.; Lucey, D.W.; Jayakumar, H.; Pudavar, H.E.; Prasad, P.N. Folate-Receptor-Mediated Delivery of InP Quantum Dots for Bioimaging Using Confocal and Two-Photon Microscopy. J. Am. Chem. Soc. 2005, 127, 11364–11371.
- Yong, K.-T.; Wang, Y.; Roy, I.; Rui, H.; Swihart, M.T.; Law, W.-C.; Kwak, S.K.; Ye, L.; Liu, J.; Mahajan, S.D.; et al. Preparation of Quantum Dot/Drug Nanoparticle Formulations for Traceable Targeted Delivery and Therapy. Theranostics 2012, 2, 681–694.
- Bagalkot, V.; Zhang, L.; Levy-Nissenbaum, E.; Jon, S.; Kantoff, P.W.; Langer, A.R.; Farokhzad, O.C. Quantum Dot−Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett. 2007, 7, 3065–3070.
- Hua, X.-W.; Bao, Y.-W.; Chen, Z.; Wu, F.-G. Carbon quantum dots with intrinsic mitochondrial targeting ability for mitochondria-based theranostics. Nanoscale 2017, 9, 10948–10960.
This entry is offline, you can click here to edit this entry!
