Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Fungi are considered terrestrial and oceans are a “fungal desert”. However, with the considerable progress made over past decades, fungi have emerged as morphologically, phylogenetically, and functionally diverse components of the marine water column. Although their communities are influenced by a plethora of environmental factors, the most influential include salinity, temperature, nutrients, and dissolved oxygen, suggesting that fungi respond to local environmental gradients.
- coastal
- pelagic
- water column
- culturable fungi
1. Current Consensus of Culturable Diversity
Traditionally, marine fungi included higher (i.e., filamentous fungi in Basidiomycota and Ascomycota) and lower (i.e., zoosporic fungi in Chytridiomycota, Oomycetes, and Labyrinthulomycetes) fungi [1]. However, the latest update on their phylogeny has grouped them into evolved branches (Ascomycota, Basidiomycota, Blastocladiomycota, and Chytridiomycota) and basal lineages (Cryptomycota, Microsporidia, and Aphelida) [2]. The first inventory of cultured marine fungi described 209 species of higher filamentous fungi, 177 species of marine-occurring yeasts, and less than 100 species of the lower marine fungi [3]. This was followed by reports of 467 [4], 530 [5], 1112 [6], and 1257 [7] species of marine fungi. Currently, about 1900 marine fungal species, distributed across seven phyla (Aphelidiomycota, Ascomycota, Basidiomycota, Blastocladiomycota, Chytridiomycota, Mucoromycota, and Microsporidia), 22 classes, 88 orders, 226 families, and 769 genera, are documented (www.marinefungi.org, accessed on 1 May 2022). Halosphaeriaceae is the largest family of marine fungi, consisting of 141 species across 59 genera, and the most specious genera are Candida (64 species), Aspergillus (47 species), and Penicillium (39 species) [6]. The documented number (ca.1900 species) is much less than the estimated 10,000 species [4], which suggests that the oceans harbor a high fungal diversity, which is yet to be fully described.
2. Mycoplankton Diversity
2.1. Microscopic Forms and Culturable Diversity
Fungi in the water column, commonly referred to as mycoplankton or planktonic fungi, were microscopically detected as individual filaments or hyphal aggregates, yeast forms, as well as picoeukaryote-associated and phytoplankton-associated zoosporic and cryptomycota forms [8][9][10][11][12]. The size range of individual filamentous forms is generally 1–3 μm in diameter and 10–200 μm in length [8][10], but in aggregate, they could reach up to 20 μm in diameter and >50 μm in length in coastal regions. The zoosporic forms (chytrids) in the coastal waters show a typical spherical sporangium (1–10 μm diameter) and rhizoid structure over 2 μm in length [13][14]. Some of these fungal forms with different lifestyles have been found to co-exist in the coastal water column [8]. The most common form of planktonic fungi encountered is yeast forms (size < 5 μm diameter), which have been found in a wide range of oceanic regions [15][16][17][18]. On the other hand, filamentous forms have been discovered mostly in coastal and coastal-upwelling regions [8][10][18].
Using culture-based methods, researchers characterized the culturable diversity of marine fungi mostly in nutrient-rich sediments. Those studies provided evidence for the presence of fungi in sediments, including subsurface, deep-sea, and anoxic sediments of different oceanic regions (Table S1). Apart from the most common ascomycetous and basidiomycetous fungi, several novel culturable fungi were also reported from marine sediments (Figure S1). Nevertheless, a vast majority of the fungi sampled from sediments are close to, or within, clades of terrestrial fungi.
Most earlier studies revealed that a large proportion of culturable diversity in the water column comprised of yeasts, including Rhodotorula, Rhodosporidium, Metchnikowia, Torulopsis, Kluyveromyces, Aureobasidium, and Cryptococcus [15][16][17][18][19]. The common filamentous fungi and molds cultured from seawaters were Aspergillus, Trichoderma, Arthrinium, Cladosporium, Penicillium, Cystobasidium, Exophiala, Graphium, Lecanicillium, Purpureocillium, Acremonium, Coniothyrium, Simplicillium, and Mucor [18][20][21][22][23]. Yeasts and filamentous fungi were even reported from extreme habitats such as the hypersaline waters of Qatar, including the halo- and psychro-tolerant, red-pigmented yeast Rhodotorula mucilaginosa, and melanized filamentous fungi Cladosporium and Alternaria [24]. Filamentous fungi were also reported from the oil-spill-contaminated marine site where the predominant genera were found to be Penicillium, Aspergillus, and Trichoderma [25].
The Ascomycota and Basidiomycota are the major phyla in both water (Figure 1) and sediment samples. The total sequence diversity of water samples was lower than that of the sediment samples, which could be a result of the poor availability of growth substrates in the water column or a low sampling effort. Interestingly, both filamentous and yeast forms of fungi were found in the global pool of culturable fungi isolated from the water column. 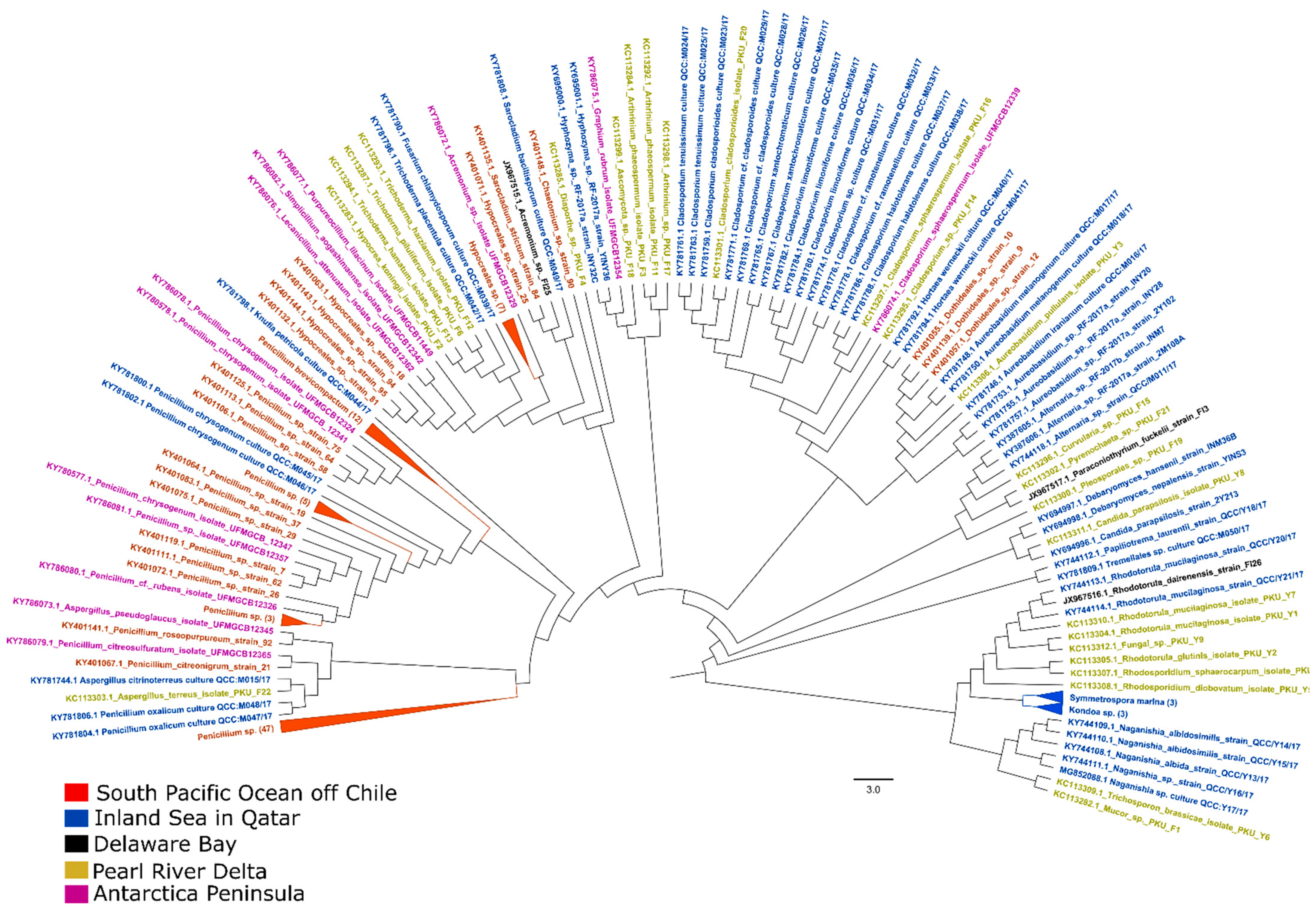

Figure 1. Maximum-likelihood (ML) tree of culturable fungi isolated from representative marine waters. A total of 192 ITS sequences of culturable fungi isolated from the water column (coastal and pelagic) across the globe were retrieved from the NCBI Nucleotide database. Sequences in the tree were aligned with MUSCLE using default settings. Phylogenetic analysis was performed using FastTree2.1 software (version 2.1, developed by Morgan N. Price, Berkeley, CA, USA) for the construction of the ML tree, which used the Shimodaira–Hasegawa test to estimate the reliability of each split in the tree. The sampling coordinates of the South Pacific Ocean off Chile [20], Inland Sea in Qatar [24], Delaware Bay [23], Pearl River Delta [21], and Antarctica Peninsula [22] are available in the corresponding publications.
Overall, culture-based studies indicate that mycoplankton diversity is limited to filamentous fungi and ascomycetous and basidiomycetous yeasts. The probable reasons for such a seemingly low diversity could be less sampling effort or nutrient-poor water column. Moreover, culture-based studies are known for their inherent biases, including the selective enrichment of a few phyla and difficulty in isolating host-associated fungi.
2.2. Molecular Diversity and Dynamics of Mycoplankton
Past culture-based studies have revealed the presence of yeasts and filamentous fungi. However, they failed to discover the zoosporic fungi in the marine water column. On the contrary, high-throughput sequencing (HTS) efforts revealed a lot more diversity, including the prevalence of zoosporic fungi, in several marine habitats [14][18][26][27][28][29]. Moreover, molecular surveys of marine eukaryotes detected fungi not only in the euphotic zone of the global ocean [30] but also in the entire water column [31]. Unfortunately, molecular surveys of eukaryotes could not provide any evidence for the extent of fungal diversity in the coastal and open-ocean waters.
Over the last decade, efforts were made to investigate the diversity of mycoplankton in both coastal and open-ocean waters (Table 1). The spatial analyses of planktonic fungi, based on DNA fingerprinting, could reveal the dynamics of positive fungal genotypes [10][32] and the presence of only Dikarya [33]. Especially in Hawaiian coastal waters, the exclusive presence of Dothideomycetes (four species) and dominance of Basidiomycota, including several novel phylotypes (42 species), were documented. The fungal communities displayed a noticeable spatial (lateral and vertical) diversity, with the vertical diversity profile being different for coastal and open-ocean waters [33]. Similarly, in the upwelling ecosystem off the coast of Central Chile, the fungal diversity was distinct, with a higher richness at the near-shore site than that of the off-shore site and a tendency to decrease with depth [32]. However, due to the inherent biases of fingerprinting techniques, this could only provide a limited view of fungal diversity. With the application of HTS, recent studies provide a deeper assessment of planktonic fungal communities and uncover many OTUs, classified into a wide range of phyla and several unclassified and possibly novel fungi from coastal waters (Table 1). Most of these studies documented the predominance of Dikarya and the prevalence of Chytridiomycota in coastal waters. However, a few studies also provided evidence for the occurrence of Cryptomycota (also known as Rozellomycota), Mucoromycota, Glomeromycota, and Neocallimastigomycota. Overall, the HTS approach provided evidence for the presence of zoosporic and basal phyla and altered the earlier notion that Dikarya fungi are exclusive inhabitants of the ocean.
Table 2. Application of high-throughput sequencing (HTS) methods in the assessment of fungal diversity of marine water columns.
| Method | Target Region | Primers | Number of OTUs | Phyla | Sampling Region |
Reference |
|---|---|---|---|---|---|---|
| 454 Pyrosequencing | 18S (V4) | TAReuk454FWD1 and TAReukREV3 | 71 | Chytridiomycota and Dikarya* | European near-shore sites | [12] |
| 454 Pyrosequencing | 18S (V4) | TAReuk454FWD1 and TAReukREV3 |
23,263 seqs. |
Chytridiomycota, Dikarya, and Cryptomycota | Arctic and temperate biomes | [34] |
| 454 Pyrosequencing | ITS | ITS1F and ITS4 |
- | Coastal water: Chytrids (36%) Open ocean: Rhizophydiales (30%) |
Tasman Sea, and East Australian Current | [35] |
| 454 Pyrosequencing | ITS1 | ITS1F and ITS2 | 3468 | Dikarya, Chytridiomycota, Mucroromycotina, and Cryptomycota | Dongchong Bay, China | [36] |
| Illumina HiSeq | ITS1 | ITS1F and ITS2 | 1483 | Dikarya, Chytridiomycota, Mucoromycota, and Cryptomycota |
Bohai Sea | [26] |
| Illumina Hiseq | ITS | 528F and 706R | 91 | Dikarya, Glomeromycota, Chytridiomycota, and Cryptomycota | Mariana Trench | [37] |
| Illumina Hiseq | ITS2 | ITS3 and ITS4 | 8701 | Dikarya, Chytridiomycota, Glomeromycota, and Rozellomycota | East China Sea water column and sediments | [29] |
| Illumina Hiseq | ITS2: | ITS3 and ITS4 | 4028 | Dikarya, Chytridiomycota, and Mucoromycota | Western Pacific Ocean (Epi-Abyssopelagic zone) | [38] |
| Illumina MiSeq | ITS | ITS1F and ITS4 | 582 | Dikarya and Chytridiomycota | Plymouth, UK | [27] |
| Illumina Miseq | ITS | ITS1F and ITS4 | 2796 | Dikarya and Chytridiomycota, Glomeromycota, and Neocallimastigomycota |
Piver’s Island Coastal Observatory (PICO), USA | [28] |
| Ion-Torrent | LSU | LR0R and EDF360R | 2305 | Ascomycota, Basidiomycota, and Chytridiomycota | Piver’s Island | [39] |
* Dikarya: Ascomycota and Basidiomycota.
Apart from the spatial variations of mycoplankton discussed above, some studies described the temporal dynamics of fungi in the coastal water column. For example, a multi-year assessment study of coastal waters at Plymouth found that Dikarya and Chytridiomycota were both dominant and dynamic, with several abundant and dominant orders [27]. Similarly, another multi-year study of fungal diversity at Piver’s Island Coastal Observatory (PICO), USA, a coastal mesotrophic ocean site, showed not only the dominance of Ascomycota but also interannually indicated seasonal patterns of Basidiomycota, Chytridiomycota, and Mucoromycotina [28]. Particularly, Chytridiomycota (order Rhizophydiales) and Mucoromycotina were detected in winter and Glomeromycota in early winter and spring. In addition, the highest richness and diversity of fungi during winter and the lowest during summer were detected at PICO. Contrastingly, in the coastal waters of the Bohai Sea, Chytridiomycota (order Rhizophydiales) dominated Ascomycota and Basidiomycota in April, indicating a possible association with phytoplankton bloom [26]. Temporal changes in the community composition of fungi were also evident during different stages of algal bloom in the coastal waters of Shenzhen [36]. Several genera prevailed in the pre-bloom stage; however, only Malassezia dominated the onset and the peak bloom stages. Saitoella and Lipomyces gradually succeeded Malassezia and eventually, Rozella dominated the terminal stage. Notably, the bloom decline stage exhibited a higher diversity than the pre-and peak-bloom stages. Collectively, the above time series studies suggest that fungi respond to seasonality and phytoplankton dynamics, which supports the view that they are residents of the coastal water column and are most likely metabolically active biomass.
Similar to the coastal water column, several lines of evidence indicated a high molecular diversity of fungi, including several unidentified and potentially novel species, in the open-ocean water column. For example, a high diversity of fungi, with the predominance of Dikarya, was reported for the first time in waters of the open-ocean transect from the Hawaiian coast to Australia [40]. Within Ascomycota and Basidiomycota, the family Nectriaceae and genus Malassezia, respectively, were the most common open-ocean fungi. Unfortunately, only Dikarya were documented, probably due to the insufficient coverage of the clone libraries. However, later studies that adopted HTS additionally uncovered several basal phyla (Table 1). For example, a study of the epi- to abyssopelagic zone of the Western Pacific Ocean documented OTUs that were assigned to Ascomycota, Basidiomycota, Chytridiomycota, and Mucoromycota, with Ascomycota as the most dominant phylum [38]. Furthermore, the classes Sordariomycetes, Eurotiomycetes, Dothideomycetes, Saccharomycetes, and the order Malasseziales were found to dominate the fungal communities. Compared to other zones, a higher OTU richness and distinct fungal community were evident in the epipelagic zone. Yet, another study of the water column suggested an increasing number of OTUs of the ascomycetous genus Aspergillus from coastal to open-ocean waters [29]. Contrastingly, in the waters of the South Pacific Ocean, Chytridiomycota (order Rhizophydiales) was reported as one of the dominant fungi. The occurrence of chytrids in oceanic waters suggested that their ecological importance in open oceans was similar to that in coastal water columns [35].
In summary, most molecular surveys of planktonic fungi report the dominance of Dikarya and suggest that many fungal OTUs in both coastal and open-ocean waters are yet to be described. Furthermore, by reprocessing more than 600 HTS datasets and analyzing 4.9 × 109 sequences (4.8 × 109 shotgun metagenomic reads and 1.0 × 108 amplicon sequences), a recent study found that every fungal phylum is represented in the global marine planktonic mycobiome [41]. However, the global marine mycobiome is generally predominated by Ascomycota, Basidiomycota, and Chytridiomycota. Particularly, the coastal and open-ocean fungal communities show the dominance of ascomycetous classes, such as Sordariomycetes, Eurotiomycetes, Dothideomycetes, Saccharomycetes, and Pezizomycetes. These findings corroborate previous culture-based studies, which report the prevalence of members of classes Dothideomycetes and Sordariomycetes in mangroves and coastal waters [42][43]. These classes of fungi are suggested to have adaptations (dispersal and attachment) for sustenance in marine environments [7][44]. Contrary to ascomycetous fungi, basidiomycetous fungi appear scarce, with Ustilaginomycetes, Agaricomycetes, Exobasidiomycetes, Wallemiomycetes, and Tremellomycetes being generally detected [26][27][45]. Interestingly, molecular surveys uncover a richer diversity of basidiomycetous classes than culture-based methods, where only Exobasidiomycetes, Agaricomycetes, and Ustilaginomycetes are described [7]. Furthermore, only Pleosporales, Dothideales, Capnodiales, Eurotiales, Malasseziales, Hypocreales, and Rhizophydiales appear ubiquitous from molecular surveys, despite the 74 known orders of culturable marine fungi [7]. The diverse and dynamic patterns of fungi in oceanic waters similar to nutrient-rich coastal waters, which emerged from molecular surveys, raise questions about their modes of nutrition and roles in oligotrophic conditions. More importantly, the differences in the abundances evident across space and time support the proposition that planktonic fungi are viable and responsive to environmental changes.
3. Environmental Drivers of Mycoplankton Diversity
Environmental factors are known to play an important role in regulating microbial community structure and diversity [46][47]. In terrestrial realms, fungi have unique requirements, and species segregate along environmental gradients [48][49]. Likewise, several lines of evidence suggest the role of environmental factors in shaping the fungal diversity of the water column (Table 2). For example, phytoplankton and primary production, nutrients, salinity, organic matter, seasonality, DO, and temperature have been reported as the key factors that govern mycoplankton diversity. In parallel, it has been suggested that riverine inputs of fungi might be responsible for a higher fungal richness in coastal sites than that in off-shore sites [32]. The other less-reported environmental factors such as ocean currents, hydrographic conditions, depth, DO, COD, nitrate, flow, conductivity, insolation, pH, DIC, oxygen concentration, riverine inputs, tidal actions, dispersal, and biological interactions were also shown to influence fungal communities of seawater columns [8][28][29][35][36][45][50][51]. These environmental associations of mycoplankton can potentially have several ecological implications, including spatiotemporal variations, organic matter decomposition, niche differentiation, host–parasite interactions, and the regulation of phytoplankton bloom (Table 2), which are yet to be fully established. Undoubtedly, the associations of fungi with a multitude of environmental factors, evident from the above studies, suggest that fungi respond to environmental gradients, and their communities can be shaped by local conditions. Although significant differences among oceanographic regions were identified, latitudinal gradients of the richness and diversity of marine fungi were not observed [41]. This was unlike the pattern observed for planktonic marine bacteria [52]. Perhaps with the availability of more HTS datasets, it would be essential to expand the collection of reference loci and genomes to determine the typical environmental drivers of planktonic fungi [41].
Table 2. Factors affecting fungal assemblages in water columns of different marine habitats and their ecological implication.
| Strongly Correlated Factors | Region | Ecological Implication | Reference |
|---|---|---|---|
| Chlorophyll a, temperature, phytoplankton biomass | Hawaiian coast | Spatial variations | [33] |
| Phytoplankton, nutrients (nitrate, phosphate, nitrite), and location | West Pacific Warm Pool | Organic matter decomposition | [40] |
| Chlorophyll a, organic matter, and warm conditions | Upwelling ecosystem off the coast of Central Chile | Organic matter decomposition | [10] |
| High nitrogen availability, reduced salinity, temperature, phytoplankton, organic matter | Coastal station off Plymouth | Temporal variations, niche differentiation, and host–parasite interactions | [27] |
| Salinity, temperature, oxygen, and nutrients | Tasman Sea, East Tasman Sea, and East Australian Current | Biogeochemical cycling and spatial variations |
[35] |
| Depth, dissolved oxygen, and nitrate | Across the globe | Local environmental conditions govern assemblages | [50] |
| Temperature, salinity, nitrate, nitrite, ammonium, and phosphate | Coastal region Dongchong Bay | Fungi regulate phytoplankton bloom | [36] |
| Temperature, depth, salinity, riverine input, location | Upwelling ecosystem off the coast of Central Chile | Organic matter decomposition | [32] |
| Dissolved nitrogen, particulate phosphorous silicate, pH, salinity, chlorophyll a | Coastal water column | Spatial variations | [26] |
| Dissolved oxygen and depth | East China Sea water and sediments | Ocean currents govern assemblages | [29] |
| Temperature, pH, insolation, dissolved inorganic carbon | Waters of Piver’s Island Coastal Observatory (PICO) |
Temporal variations | [28] |
| Depth, temperature, and dissolved oxygen | Epi- to abyssopelagic zones of the Western Pacific Ocean | Distinct zonation of assemblages in the water column | [38] |
| Salinity | Baltic Sea | Salinity threshold separates assemblages | [53] |
This entry is adapted from the peer-reviewed paper 10.3390/jof8050491
References
- Raghukumar, S. The Pelagic Ecosystem. In Fungi in Coastal and Oceanic Marine Ecosystems: Marine Fungi; Raghukumar, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 185–217.
- Pang, K.-L.; Jones, E.B.G. Phylogenetic Diversity of Fungi in the Sea including the Opisthosporidia. In Biology of Microfungi; Li, D.-W., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 267–283.
- Kohlmeyer, J.; Kohlmeyer, E. 1—Introduction. In Marine Mycology; Academic Press: Cambridge, MA, USA, 1979; pp. 1–6.
- Kis-Papo, T. Marine fungal communities. In The Fungal Community: Its Organization and Role in the Ecosystem; Dighton, J., White, J.F., Oudemans, P., Eds.; Taylor & Francis: Boca Raton, NJ, USA, 2005; pp. 61–92.
- Jones, E.B.G.; Sakayaroj, J.; Suetrong, S.; Somrithipol, S.; Pang, K.L. Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers. 2009, 35, 1–187.
- Jones, E.B.G.; Suetrong, S.; Sakayaroj, J.; Bahkali, A.H.; Abdel-Wahab, M.A.; Boekhout, T.; Pang, K.-L. Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers. 2015, 73, 1–72.
- Jones, E.B.G.; Pang, K.-L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J.; et al. An online resource for marine fungi. Fungal Divers. 2019, 96, 347–433.
- Li, Q.; Wang, X.; Liu, X.; Jiao, N.; Wang, G. Diversity of parasitic fungi associated with phytoplankton in Hawaiian waters. Mar. Biol. Res. 2016, 12, 294–303.
- Jones, M.D.M.; Forn, I.; Gadelha, C.; Egan, M.J.; Bass, D.; Massana, R.; Richards, T.A. Discovery of novel intermediate forms redefines the fungal tree of life. Nature 2011, 474, 200.
- Gutiérrez, M.H.; Pantoja, S.; Quiñones, R.A.; González, R.R. First record of filamentous fungi in the coastal upwelling ecosystem off central Chile. Gayana (Concepción) 2010, 74, 66–73.
- Lepere, C.; Ostrowski, M.; Hartmann, M.; Zubkov, M.V.; Scanlan, D.J. In situ associations between marine photosynthetic picoeukaryotes and potential parasites—A role for fungi? Environ. Microbiol. Rep. 2016, 8, 445–451.
- Richards, T.A.; Leonard, G.; Mahé, F.; Del Campo, J.; Romac, S.; Jones, M.D.M.; Maguire, F.; Dunthorn, M.; De Vargas, C.; Massana, R.; et al. Molecular diversity and distribution of marine fungi across 130 European environmental samples. Proc. R. Soc. B: Biol. Sci. 2015, 282, 20152243.
- Gutiérrez, M.H.; Jara, A.M.; Pantoja, S. Fungal parasites infect marine diatoms in the upwelling ecosystem of the Humboldt current system off central Chile. Environ. Microbiol. 2016, 18, 1646–1653.
- Hassett, B.T.; Gradinger, R. Chytrids dominate arctic marine fungal communities. Environ. Microbiol. 2016, 18, 2001–2009.
- Fell, J.W. Yeasts in marine environments. In Marine Fungi and Fungal-Like Organisms; Jones, E.B.G., Pang, K.L., Eds.; Walter de Gruyter GmbH & Co. KG: Berlin, Germany; Boston, MA, USA, 2012; pp. 91–102.
- Kutty, S.N.; Philip, R. Marine yeasts-a review. Yeast 2008, 25, 465–483.
- Fotedar, R.; Chatting, M.; Kolecka, A.; Zeyara, A.; Al Malki, A.; Kaul, R.; Bukhari, S.J.; Moaiti, M.A.; Febbo, E.J.; Boekhout, T.; et al. Communities of culturable yeasts and yeast-like fungi in oligotrophic hypersaline coastal waters of the Arabian Gulf surrounding Qatar. Antonie Van Leeuwenhoek 2022, 115, 609–633.
- Pham, T.T.; Dinh, K.V.; Nguyen, V.D. Biodiversity and Enzyme Activity of Marine Fungi with 28 New Records from the Tropical Coastal Ecosystems in Vietnam. Mycobiology 2021, 49, 559–581.
- Roth, F.J.; Orpurt, P.A.; Ahearn, D.G. Occurrence and distribution of fungi in a subtropical marine environment. Can. J. Bot. 1964, 42, 375–383.
- Vera, J.; Gutiérrez, M.H.; Palfner, G.; Pantoja, S. Diversity of culturable filamentous Ascomycetes in the eastern South Pacific Ocean off Chile. World J. Microbiol. Biotechnol. 2017, 33, 1–13.
- Li, L.; Singh, P.; Liu, Y.; Pan, S.; Wang, G. Diversity and biochemical features of culturable fungi from the coastal waters of Southern China. AMB Express 2014, 4, 60.
- Gonçalves, V.N.; Vitoreli, G.A.; de Menezes, G.C.A.; Mendes, C.R.B.; Secchi, E.R.; Rosa, C.A.; Rosa, L.H. Taxonomy, phylogeny and ecology of cultivable fungi present in seawater gradients across the Northern Antarctica Peninsula. Extremophiles 2017, 21, 1005–1015.
- Burgaud, G.; Woehlke, S.; Rédou, V.; Orsi, W.; Beaudoin, D.; Barbier, G.; Biddle, J.F.; Edgcomb, V.P. Deciphering the presence and activity of fungal communities in marine sediments using a model estuarine system. Aquat. Microb. Ecol. 2013, 70, 45–62.
- Fotedar, R.; Kolecka, A.; Boekhout, T.; Fell Jack, W.; Al-Malki, A.; Zeyara, A.; Al Marri, M. Fungal diversity of the hypersaline Inland Sea in Qatar. Bot. Mar. 2018, 61, 595.
- Bovio, E.; Gnavi, G.; Prigione, V.; Spina, F.; Denaro, R.; Yakimov, M.; Calogero, R.; Crisafi, F.; Varese, G.C. The culturable mycobiota of a Mediterranean marine site after an oil spill: Isolation, identification and potential application in bioremediation. Sci. Total Environ. 2017, 576, 310–318.
- Wang, Y.; Sen, B.; He, Y.; Xie, N.; Wang, G. Spatiotemporal Distribution and Assemblages of Planktonic Fungi in the Coastal Waters of the Bohai Sea. Front. Microbiol. 2018, 9, 584.
- Taylor, J.D.; Cunliffe, M. Multi-year assessment of coastal planktonic fungi reveals environmental drivers of diversity and abundance. ISME J. 2016, 10, 2118–2128.
- Duan, Y.; Xie, N.; Song, Z.; Ward, C.S.; Yung, C.-M.; Hunt, D.E.; Johnson, Z.I.; Wang, G. A High-resolution Time-series Reveals Seasonal Patterns of Planktonic Fungi at a Temperate Coastal Ocean Site (Beaufort, North Carolina, USA). Appl. Environ. Microbiol. 2018, 84, e00967-18.
- Li, W.; Wang, M.; Pan, H.; Burgaud, G.; Liang, S.; Guo, J.; Luo, T.; Li, Z.; Zhang, S.; Cai, L. Highlighting patterns of fungal diversity and composition shaped by ocean currents using the East China Sea as a model. Mol. Ecol. 2018, 27, 564–576.
- Vargas, C.D.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Bescot, N.L.; Probert, I. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 1261605.
- Xu, D.; Li, R.; Hu, C.; Sun, P.; Jiao, N.; Warren, A. Microbial Eukaryote Diversity and Activity in the Water Column of the South China Sea Based on DNA and RNA High Throughput Sequencing. Front. Microbiol. 2017, 8, 1121.
- Gutiérrez, M.H.; Garcés, D.V.; Pantoja, S.; González, R.R.; Quiñones, R.A. Environmental fungal diversity in the upwelling ecosystem off central Chile and potential contribution to enzymatic hydrolysis of macromolecules in coastal ecotones. Fungal Ecol. 2017, 29, 90–95.
- Gao, Z.; Johnson, Z.I.; Wang, G. Molecular characterization of the spatial diversity and novel lineages of mycoplankton in Hawaiian coastal waters. ISME J. 2010, 4, 111–120.
- Comeau, A.M.; Vincent, W.F.; Bernier, L.; Lovejoy, C. Novel chytrid lineages dominate fungal sequences in diverse marine and freshwater habitats. Sci. Rep. 2016, 6, 30120.
- Jeffries, T.C.; Curlevski, N.J.; Brown, M.V.; Harrison, D.P.; Doblin, M.A.; Petrou, K.; Ralph, P.J.; Seymour, J.R. Partitioning of fungal assemblages across different marine habitats. Environ. Microbiol. Rep. 2016, 8, 235–238.
- Sun, J.Y.; Song, Y.; Ma, Z.P.; Zhang, H.J.; Yang, Z.D.; Cai, Z.H.; Zhou, J. Fungal community dynamics during a marine dinoflagellate (Noctiluca scintillans) bloom. Mar. Environ. Res. 2017, 131, 183–194.
- Wang, Z.-P.; Liu, Z.-Z.; Wang, Y.-L.; Bi, W.-H.; Liu, L.; Wang, H.-Y.; Zheng, Y.; Zhang, L.-L.; Hu, S.-G.; Xu, S.-. Set al. Fungal community analysis in seawater of the Mariana Trench as estimated by Illumina HiSeq. RSC Adv. 2019, 9, 6956–6964.
- Li, W.; Wang, M.; Burgaud, G.; Yu, H.; Cai, L. Fungal Community Composition and Potential Depth-Related Driving Factors Impacting Distribution Pattern and Trophic Modes from Epi- to Abyssopelagic Zones of the Western Pacific Ocean. Microb. Ecol. 2019, 78, 820–831.
- Picard, K.T. Coastal marine habitats harbor novel early diverging fungal diversity. Fungal Ecol. 2017, 25, 1–13.
- Wang, X.; Singh, P.; Gao, Z.; Zhang, X.; Johnson, Z.I.; Wang, G.Y. Distribution and diversity of planktonic fungi in the West Pacific Warm Pool. PLoS ONE 2014, 9, e101523.
- Hassett, B.T.; Vonnahme, T.R.; Peng, X.; Jones, E.B.G.; Heuzé, C. Global diversity and geography of planktonic marine fungi. Bot. Mar. 2020, 63, 121–139.
- Jones, E.B.G.; Pang, K.-L. Tropical aquatic fungi. Biodivers. Conserv. 2012, 21, 2403–2423.
- Jones, E.B.G. Ultrastructure and taxonomy of the aquatic ascomycetous order Halosphaeriales. Can. J. Bot. 1995, 73, 790–801.
- Suetrong, S.; Schoch, C.L.; Spatafora, J.W.; Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; Sakayaroj, J.; Phongpaichit, S.; Tanaka, K.; Hirayama, K.; Jones, E.B.G. Molecular systematics of the marine Dothideomycetes. Stud. Mycol. 2009, 64, 155–173.
- Wang, Y.; Sen, K.; He, Y.; Xie, Y.; Wang, G. Impact of environmental gradients on the abundance and diversity of planktonic fungi across coastal habitats of contrasting trophic status. Sci. Total Environ. 2019, 683, 822–833.
- Gilbert, J.A.; Steele, J.A.; Caporaso, J.G.; Steinbruck, L.; Reeder, J.; Temperton, B.; Huse, S.; McHardy, A.C.; Knight, R.; Joint, I.; et al. Defining seasonal marine microbial community dynamics. ISME J. 2012, 6, 298–308.
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541.
- Taylor, D.L.; Hollingsworth, T.N.; McFarland, J.W.; Lennon, N.J.; Nusbaum, C.; Ruess, R.W. A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol. Monogr. 2014, 84, 3–20.
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438.
- Tisthammer, K.H.; Cobian, G.M.; Amend, A.S. Global biogeography of marine fungi is shaped by the environment. Fungal Ecol. 2016, 19, 39–46.
- Xu, W.; Luo, Z.-H.; Guo, S.; Pang, K.-L. Fungal community analysis in the deep-sea sediments of the Pacific Ocean assessed by comparison of ITS, 18S and 28S ribosomal DNA regions. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 109, 51–60.
- Fuhrman, J.A.; Steele, J.A.; Hewson, I.; Schwalbach, M.S.; Brown, M.V.; Green, J.L.; Brown, J.H. A latitudinal diversity gradient in planktonic marine bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 7774–7778.
- Rojas-Jimenez, K.; Wurzbacher, C.; Jürgens, K.; Labrenz, M.; Grossart, H.-P. A Salinity Threshold Separating Fungal Communities in the Baltic Sea. Front. Microbiol. 2019, 10, 680.
This entry is offline, you can click here to edit this entry!
