Extracellular vesicles (EVs) are small membrane vesicles released by all cells and involved in intercellular communication. Importantly, EVs cargo includes nucleic acids, lipids, and proteins constantly transferred between different cell types, contributing to autocrine and paracrine signaling. In recent years, they have been shown to play vital roles, not only in normal biological functions, but also in pathological conditions, such as cancer. In the multistep process of cancer progression, EVs act at different levels, from stimulation of neoplastic transformation, proliferation, promotion of angiogenesis, migration, invasion, and formation of metastatic niches in distant organs, to immune escape and therapy resistance. Moreover, as products of their parental cells, reflecting their genetic signatures and phenotypes, EVs hold great promise as diagnostic and prognostic biomarkers. Importantly, their potential to overcome the current limitations or the present diagnostic procedures has created interest in bladder cancer (BCa). Indeed, cystoscopy is an invasive and costly technique, whereas cytology has poor sensitivity for early staged and low-grade disease. Several urine-based biomarkers for BCa were found to overcome these limitations.
- extracellular vesicles
- microvesicles
- bladder cancer
- urothelial cancer
- biomarkers
- liquid biopsy
- Introduction: Bladder Cancer and Disease Management
Urological tumors represent approximately 25% of all human cancers [1]. Bladder cancer (BCa) is the 10th most common, and the 9th cause of death by malignancy worldwide [2]. Aging, ethnicity, and male gender are considered non-modifiable risk factors [2–4], but most tumors are derived from acquired environmental exposure to carcinogenic substances. Cigarette smoking is considered the main risk factor [5,6], with an estimated causal association for half of BCa in both genders [7–9], whereas occupational exposure accounts for 10–20% of all cases [10]. The worldwide incidence of BCa seems to reflect areas with higher exposure to risk factors [10], which explains why developed countries have a larger number of diagnosed cases [2,10]. As an example, Schistosoma haematobium infection, another known risk factor, is more prevalent in northern and sub-Saharan African countries, where there is a relatively higher incidence of BCa [10]. In addition, differences in healthcare systems also account for disparity of incidence rates, being better resources associated to an easier and faster diagnosis.
Urothelial cancer originates in the epithelial cells of the urothelium, extending from the renal pelvis to the urethra [11–13]. The majority of these tumors are located in the bladder, accounting for 90–95% of cases, while 5–10% are located in the upper urinary tract (UUT) [14–18]. Tumor extension is classified according to the TNM (Tumor-Node-Metastasis) staging system. At diagnosis, approximately 75–80% of bladder tumors are non-muscle invasive (NMIBC), which includes mucosa (for stages Ta and Cis) and lamina propria (T1 stage) confined disease, while 20–25% are muscle-invasive (MIBC), when invading the muscle layer and beyond (T2–T4 stages) [1,4,14].
Although clinical presentation may be suggestive, the gold standard diagnostic procedures are cystoscopy and urinary cytology [19–24]. Nevertheless, cystoscopy is an invasive, operator-dependent procedure, with low sensitivity for small papillary or Cis tumors, which, if underdiagnosed and untreated, progress to muscle-invasive disease in approximately half of the patients [19–24]. The sensitivity and specificity of white light cystoscopy is 71% (95% CI: 0.49–0.93%) and 72% (95% CI: 47–96%), respectively [24]. However, due to its invasiveness, it is frequently associated with side effects such as dysuria (50%), hematuria (19%), or urinary tract infection (3%) [25,26].
As for urinary cytology, it has high diagnostic accuracy for high grade lesions and Cis, with a sensitivity of 80–90% and specificity between 98% and 100% [27]. However, it exhibits low sensitivity for low grade lesions, between 4% and 31% [28–33], and high rate of false positives, due to benign or inflammatory conditions produced by chemo or radiation therapy [34,35]. To overcome these limitations, several urinary biomarkers were developed and are currently commercially available. Compared to cytology, they have higher sensitivity but lower specificity and are, unfortunately, less useful in low risk BCa [36–38]. Therefore, consensus among the different international societies on these biomarkers still do not recommend them as replacements of cytology in the current clinical practice [36–38].
The standard therapy for NMIBC is trans-urethral resection of the bladder (TURB), with both diagnostic and therapeutic purposes, complemented or not by intravesical adjuvant treatment [39,40]. However, even after complete endoscopic resection, there is a high recurrence rate, around 50–70%, and 10–30% will progress to MIBC [39,40]. This feature of BCa natural history elicits the need for a regular follow-up with cystoscopy and cytology at every 3 months interval, generally accompanied by repeated treatments due to recurrence, and which frequently result in high morbidity and economic burden [1,41,42].
Driven by the invasiveness and morbidity of cystoscopy, the lack of acceptable sensitivity of urinary cytology and of specificity of the commercially available urinary diagnostic biomarkers, urge the need for extensive research on the identification of novel and more effective biomarkers, to implement better tools for diagnosis, follow-up, and screening of at risk populations [1,29,34,42–44].
Extracellular vesicles (EVs) are small membrane vesicles which have emerged as a source of biomarkers in bladder cancer [45]. Their detection in liquid biopsies is feasible, due to their presence and stability in most human fluids, and may serve as biomarkers in bladder cancer early detection as they present similar cargo to their donor cancer cells [46]. Additionally, they have some advantages as a source of biomarkers since they are more abundant in liquid biopsies compared to circulating tumor cells (CTCs), protect their cargo against degradation and may carry molecular signatures associated with specific phenotypes [47–49]. The present review focus on the status of urinary biomarkers in diagnosis and follow-up of bladder cancer, pinpointing the emerging potential role of urinary EVs on bladder cancer diagnosis and management.
- Liquid Biopsy as a Source of Biomarkers for Bladder Cancer
The ideal biomarker would be cost-effective, objective, fast to process, and easy to interpret, with high sensitivity and specificity [43,50–54]. For urothelial cancer biomarkers, four goals have been proposed to be accomplished: (i) reduce the need for frequent invasive procedures; (ii) exclude recurrence; (iii) detect progression towards invasive disease; (iv) predict effective treatment response [43,44]. The close contact with urothelium makes urine an attractive approach to detect the presence of tumor cells, in a minimally invasive way. Importantly, this liquid biopsy approach would allow multiple longitudinal sampling of the tumor to identify presence of malignancy, grade, and genomic landscape for improved clinical follow-up. [53,54].
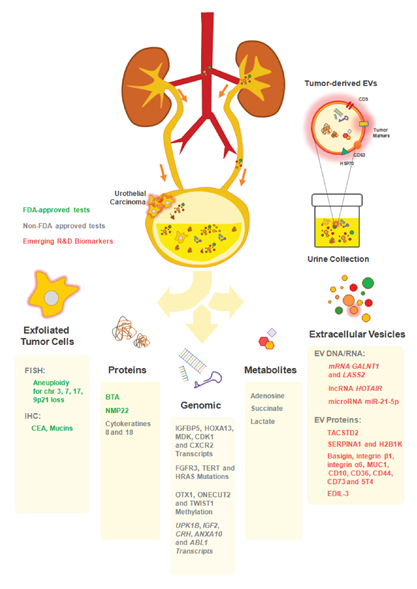
Following this line of thought, previous research for BCa biomarkers has been conducted using mostly proteins, nucleic acids, inflammatory and metabolite markers, within the concept of liquid biopsies [55,56]. Taking into consideration that such biopsies concern the detection of any kind of molecular or cellular biomarkers in patient bodily fluids (including urine, blood, saliva, pleural, peritoneal, or cerebrospinal fluids), a novel biomarkers array emerged. These include circulating tumor cells (CTCs), proteins, metabolites, circulating nucleic acids, namely cell-free tumor DNA (ctDNA), messenger RNA (mRNA), micro RNA (miRNA), or long non-coding RNA (lncRNA). Most of these biomarkers may be found free or within extracellular vesicles (EVs) shed by tumor cells or by other elements of the tumor microenvironment [56,57] (Figure 1). There is a growing interest on the liquid biopsy concept, since (i) the biomarkers found have extensive potential for diagnosis and monitoring of disease stage and recurrence; (ii) prediction of therapeutic response/resistance and disease prognosis, with minimally invasive procedures, and (iii) helping therapeutic clinical reasoning based on identified molecular changes [56–58].
Figure 1. Urine biomarkers for bladder cancer (BCa) diagnosis and follow up. Illustration of the distinct available approaches for the detection of urothelial cancer cells in patients’ urine. The close interaction between the bladder tumor and the urine makes this body fluid a reliable source of cancer biomarkers. A plethora of non-invasive assays exploring distinct analytes (exfoliated tumor cells; proteins; genes; metabolites and extracellular vesicles) in patients’ urine allows the longitudinal analysis of tumor progression. Some of the commercially-available tests includes FDA-approved (UroVysion™: aneuploidy of chromosomes 3; 7; 17 and the loss of 9p21 by FISH; ImmunoCyt™/uCyt+™ test: detection of carcinoembrionary antigen (CEA) and mucins by immunohistochemistry (IHC); bladder tumor antigen (BTA) TRAK/BTA Stat and NMP22 BC test kit); non-FDA approved (CxBladder™: IGFBP5, HOXA13, MDK, CDK1 and CXCR2 by RT-qPCR; Assure MDx™: FGFR3, TERT and HRAS (mutations), OTX1, ONECUT2 and TWIST1 (methylation); XPert Bladder Cancer Monitor™: UPK1B, IGF2, CRH, ANXA10 and ABL1 by RT-qPCR; and UBC™: cytokeratins 8 and 18 by ELISA) and the emerging extracellular vesicles (EV)-based biomarkers (not commercialized yet).
2.1. Commercially Available Urine Biomarkers in Bladder Cancer
Several interesting and promising biomarkers have been under clinical scrutiny during the past years, although only those approved by in vitro diagnostics (IVD) regulatory entities (e.g., FDA) became commercially available biomarkers, to be used as adjuncts to cystoscopy in primary diagnosis and follow-up of BCa. Taking into consideration the various reports on the subject, novel urinary biomarkers contributed to higher sensitivity but lower specificity than cytology, leaving them out of international guidelines recommendations [1,36,53,54,59–62]. Table 1 provides an overview of the biomarkers approved for clinical use and their reported diagnostic accuracy.
Table 1. Urine-based tests to aid bladder cancer clinical reasoning.
|
Test |
Sample |
Biomarker |
Assay |
Purpose |
Sensitivity |
Specificity |
References |
|
BTA TRAK® |
Protein |
Complement factor H-related |
CIA |
Follow-Up |
0.64 (0.58–0.69) |
0.77 (0.73–0.81) |
[63–65] |
|
BTA Stat ® |
Protein |
Complement factor H-related |
SIA |
Follow-Up |
0.65 (0.54–0.75) |
0.74 (0.64–0.82) |
[64–66] |
|
NMP22 BC test® |
Protein |
NMP-22 |
SIA |
Follow-Up |
0.69 (0.62–0.75) |
0.77 (0.70–0.83) |
[67,68] |
|
NMP22 BladderChek test® |
Protein |
NMP-22 |
SIA |
Diagnosis |
0.47 (0.33–0.61) |
0.93 (0.81–0.97) |
[67–69] |
|
Follow-Up |
0.70 (0.40–0.89) |
0.83 (0.75–0.89) |
[67–69] |
||||
|
ImmunoCyt/uCyt+™ |
Sediment |
Tumor associated cellular antigens (M344; LDQ10; 19A11) |
IF cytology |
Diagnosis |
0.85 (0.78–0.90) |
0.83 (0.77–0.87) |
[64,65,70] |
|
Follow-Up |
0.75 (0.64–0.83) |
0.76 (0.70–0.81) |
[64,65,70] |
||||
|
UroVysion™ |
Sediment |
Aneuploidy for chromosomes 3; 7; 17; and loss of 9p21 locus |
FISH |
Diagnosis |
0.73 (0.50–0.88) |
0.95 (0.87–0.98) |
[71–73] |
|
Follow-Up |
0.55 (0.36–0.72) |
0.80 (0.66–0.89) |
[71–73] |
||||
|
CxBladder Detect® |
mRNA |
IGFBP5; HOXA13; MDK; CDK1; CXCR2 |
RT-qPCR |
Diagnosis |
0.74 (0.65–0.81) |
0.82 (0.79–0.84) |
[74] |
|
CxBladder Monitor® |
mRNA |
IGFBP5; HOXA13; MDK; CDK1; CXCR2 |
RT-qPCR |
Follow-Up |
0.91 (0.88–0.99) |
0.96 (NPV) |
[75] |
|
AssureMDx™ |
DNA |
FGFR3; TERT; HRAS; OTX1; ONECUT2; TWIST1 |
DNA methylat |
Diagnosis |
0.93 |
0.86 |
[76] |
|
Xpert® Bladder Cancer |
mRNA |
UPK1B; IGF2; CRH; ANXA10; ABL1 |
RT-qPCR |
Follow-Up |
0.84 (0.69–0.93) |
0.91 (0.83–0.96) |
[77] |
|
UBC® |
Protein |
Cytokeratin 8 and 18 fragments |
SIA |
Diagnosis |
0.61–0;65 |
0.77–0.82 |
[78] |
Abbreviations: BTA; bladder tumor antigen; CIA; colorimetric immunoassay; IF; immunofluorescence; NMP; nuclear matrix protein; UBC; urinary bladder cancer antigen; FISH; fluorescence in situ hybridization; RT-qPCR; reverse transcription-quantitative polymerase chain reaction; SIA; sandwich immunoassay
2.1.1. FDA-Approved Urine Biomarkers
The bladder tumor antigen (BTA) is a complement factor H related protein secreted by malignant cells, which confers them survival advantage, as it interferes in the complement cascade [79]. There are two approved versions of this test for BCa follow-up in concurrent use with cystoscopy, the BTA TRAK and the BTA Stat (Polymedco Inc., Cortlandt Manor, New York, USA) [63]. In different reviews and meta-analysis, the BTA Stat has a sensitivity and specificity of 64% and 77%, respectively, whereas the BTA Trak has 65% and 74%, respectively [64–66]. The sensitivity was higher in the diagnosis of symptomatic patients rather than in follow-up, but with similar specificity. Both tests demonstrated higher sensitivity than urinary cytology, despite the decreased specificity in conditions where the complement factor H related protein is present, such as in other genitourinary malignancies and benign conditions with hematuria, including lithiasis, inflammation, instrumentation, and intra-vesical therapies [31,64–66,80].
The nuclear matrix has an important role on DNA replication and RNA transcription and splicing [81], with nuclear matrix proteins (NMP) being essential components of mitosis, with a role in tumoral proliferation. Numerous NMPs have been described in solid tumors, although NMP22 was shown to be specific for BCa [81,82]. It is released from apoptotic cells towards urine, with significantly higher release rate in cancer than in normal cells [81,83,84]. The NMP22 BC test kit (Matritech Inc.; Newton, MA, USA) is a quantitative test used for patient follow-up, whereas the NMP22 BladderChek test® (Matritech Inc.; Newton, MA, USA) is qualitative and used for both follow-up and initial diagnosis, in symptomatic patients [85–87]. Concerning sensitivity and specificity, the quantitative test has 69% and 77%, while the qualitative has 58% and 88%, respectively [64,67–69,88,89]. When compared to urinary cytology, the sensitivity of NMP22 was higher (70% versus 40%), albeit specificity was lower (81% versus 97%) [28]. Taken together, both NMP22 and cytology, resulted in sensitivity of 91% [28,89]. Notably, Grossman et al. studied approximately 2000 patients, to compare NMP22 Bladder Chek test® with cystoscopy, and observed decreased NMP22 sensitivity (50–56%) in comparison to cystoscopy (89–91%), although diagnostic accuracy was 94–99% if both tests were considered together [90]. Although NMP22 has higher sensitivity than urinary cytology, specificity is too low to replace it. The fact that it is released from apoptotic cells, which might also be seen in benign conditions, is responsible for the relatively high false positive rate [91]. However, if combined with cistoscopy, this significantly increases its diagnostic value.
The ImmunoCyt™/uCyt+™ test (Diagnocure Inc, Quebec, Canada) combines cytology with monoclonal antibody immunofluorescence labelling to detect three BCa antigens, M344, LDQ10, and 19A11, specifically found in malignant exfoliated urothelial cells [92]. To be positive, it requires many exfoliated cells (>500 per field). This test is expensive, with inter-observer variation and time-consuming analysis, but less prone to be influenced by benign inflammatory conditions, comparatively to other tests [93,94]. It is recommended in BCa patients only for follow-up as adjunct test to urinary cytology [95]. Sensitivity varies between 83% and 85% and specificity between 75% and 87%. These are higher in primary diagnosis than follow-up [28,64,65,70]. Mowatt et al. [28] compared uCyt+™ with urinary cytology and showed that this test presented higher sensitivity (82% versus 44%) and lower specificity (85% versus 94%), respectively. Interestingly, the simultaneous use of both tests improved sensitivity without impacting on specificity (87% and 68%, respectively). Sensitivity and specificity, in the study of Schmitz-Dräger et al. [96], was 85% and 88% for immunocytology and 84% and 98% for cystoscopy. When combined, sensitivity increased to 100%, whereas specificity decreased to 87%. Although less prone to interference, immunoCyt™ has lower specificity than urinary cytology. Likewise, despite combination with cystoscopy increases sensitivity, the false positive rate remains elevated [96]. Pfister et al. [97] assert that due to its good sensitivity, the combined use of uCyt+™ with cytology might delay the time intervals between cystoscopies, particularly in lower risk patients. Currently, this test was approved only for patient follow-up [95].
UroVysion (Abbott Laboratories, Abbott Park, Illinois, USA) is a fluorescence in situ hybridization (FISH) probe set to detect bladder cancer cells [95,98]. It uses fluorescent labelled DNA probes to assess genetic changes in exfoliated cells, namely chromosomal aberrations suggestive of BCa, aneuploidy of chromosomes 3, 7, and 17, and loss of the 9p21 locus. It has been approved for primary diagnosis and follow-up of BCa patients [95,98]. The reported sensitivity is 63–72% and specificity 85–87% [28,64]. Their diagnostic accuracy was superior in primary diagnosis than in follow-up, showing low sensitivity, similarly to urinary cytology, particularly for low grade tumors [71,72]. Compared to cytology, UroVysion had better sensitivity (72% vs. 42%) and lower specificity (83% vs. 96%) [71]. When used simultaneously, there was a significant improvement in sensitivity but still a low specificity of 50% [72,73]. UroVysion™ is more expensive than cytology and requires specialized laboratory techniques. However, it could be useful in situations of atypical cytology and equivocal cystoscopy, identifying patients that may need further investigation [62,97]. Two prospective studies found that UroVysion had high positive predictive value, supporting that patients with a positive test and negative cystoscopy are more likely to have disease recurrence within one year [99–101]. Thus, a FISH test that is positive may be used to anticipate BCa recurrence during follow-up, especially in low risk patients [99,100], and reduce the number of unnecessary bladder biopsies [102]. Therefore, these studies suggest that chromosomic aberrations precede the detection of malignant lesions by cystoscopy and other standard techniques [101].
Analyses comparing the above-mentioned biomarkers have been reported. No differences were found in terms of sensitivity and specificity between the NMP22 test kit (cut-off > 10 U/mL) and the BTA Stat, in different stages and tumor grades [91,103–108]. The ImmunoCyt™ has higher sensitivity for low stage (Ta, T1) and low-grade tumors, although lower specificity than the UroVysion™ test [107,109,110]. However, although these tests were FDA approved for diagnosis and follow-up of BCa, together with standard techniques, most of these studies are case–control ones in populations with high prevalence of the disease, giving them an unrealistically high positive predictive value. On the other hand, the question remains how to interpret positive findings of these tests, when no significant findings are found on cystoscopy during follow-up. In fact, most positive results have not been submitted to confirmatory biopsy. Moreover, there are few external validation studies to support their use in daily practice. In summary, multicentric prospective studies are required to assess consequences from positive and negative tests in the long term, to increase the likelihood to be supported by international urology organizations.
2.1.2. Non-FDA Approved Urine Biomarkers
To overcome the limitations of approved diagnostics biomarkers, extensive research is ongoing to find more effective biomarkers for BCa diagnosis and follow-up. There are several commercially available tests, despite not being approved by regulatory institutions. The CxBladder (Pacific Edge Diagnostics, Dunedin, New Zeland) is a RTqPCR test in voided urine, that quantifies different mRNAs expressed in BCa, as IGFBP5, HOXA13, MDK, CDK1, and CXCR2, associated with non-malignant conditions, to reduce the number of false-positives results due to inflammation [111,75]. The Triage™, Detect™, and Monitor™ tests have specific population targets. The first was developed for screening of high-risk populations as a pre-test guiding the need for cystoscopy, the Cxbladder Detect™ was intended for aiding in diagnosis of symptomatic patients and the Monitor™ for BCa follow-up [75]. Studies using Cxbladder Detect™ found higher sensitivity but lower specificity than cytology (73.6% sensitivity and 81.7% specificity) in one study [74], while another described 82% sensitivity and similar specificity [112]. There are reports for Cxbladder Monitor™ stating a sensitivity of 93% that increases to 95% in high risk patients [75]. A large study comparing biomarkers performance for BCa detection in urine, found that the Cxbladder Monitor™ sensitivity (91%) overcomed cytology by 22%, NMP22 BC test kit® by 26% and NMP22 BladderChek® by 11%, with an estimated reduction in the number of cystoscopies needed in follow-up by 81.7% [38]. Although prospective confirmatory trials are needed, some authors suggest its use as an auxiliary test to postpone the need of repeated cystoscopies in low risk patients [38,54]. The Assure MDx™ (MDx Health, Irvine, CA, USA) is a test performed in urine to identify DNA mutations in three genes (FGFR3, TERT, and HRAS) and methylations in another three genes (OTX1, ONECUT2, and TWIST1) [113]. A multicentric study demonstrated a sensitivity of 93% and specificity of 86% for BCa diagnosis [76]. It might be useful for screening low risk patients with symptomatic hematuria, reducing by an estimated 77% the number of unnecessary diagnostic cystoscopies. The XPert® Bladder Cancer (BC) Monitor (Cepheid, Sunnyvale, California, USA) is a RT-PCR test that measures the number of urinary transcripts in five genes, UPK1B, IGF2, CRH, ANXA10, and ABL1, and was designed for BCa patient follow-up [77]. This test was superior to cytology on NMIBC during follow-up, in terms of sensitivity (84% versus 33%), while presenting similar specificity (91% versus 94%) [77], despite controversial findings from another study, that indicated 46.7% sensitivity and 77% specificity [114]. The heterogeneity between studies and the lack of external validation makes its present use unreliable. The UBC® (Urinary bladder cancer) is a test that detects the expression of cytokeratins 8 and 18 in urine, with presentation of quantitative, UBC®-ELISA, and qualitative UBC®-rapid procedures [78]. The reported sensitivity for UBC®-rapid was 86.9% for detecting Cis, 30.4% for low grade NMIBC, 71.4% for high grade NMBC, and 60% for MIBC [78]. Other studies reported sensitivities between 30% and 87% for Cis and specificity of 63–91% [115–118]. The UBC®-rapid is a test that provides results within 10 min, but in comparison with other tests has the lowest specificity [115].
2.2. Emerging Urine Biomarkers
Besides these commercially available diagnostic tools for BCa detection in the urine, extensive research is underway to find more effective biomarkers [86,119,120]. The insufficient number of patients in most studies, the lack of external validation in large scale prospective studies, and absence of comparative trials between biomarkers, foster the need for both methodological improvement of existing biomarkers and uncover novel robust biomarkers. Moreover, the existing biomarkers, in general, perform poorly in low risk BCa or have low specificity, and are more accurate in the initial diagnosis of BCa than in follow-up [66]. Taken together, these limitations preclude actual recommendations by most international clinical societies, and current literature suggests that single biomarkers are insufficient to overcome this problem. Therefore, the current trends of research are focusing on the combination of different biomarker signatures, to develop more accurate diagnostic and surveillance tools in BCa, as well as to predict its behavior in order to provide prognostic information [121].
This entry is adapted from the peer-reviewed paper 10.3390/cancers12061400