Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cerebral venous thrombosis (CVT) is a relatively rare disorder in the general population and is frequently misdiagnosed upon initial examination. The knowledge of wide clinical aspects and imaging signs will be essential in providing a timely diagnosis.
- cerebral veins and dural sinuses thrombosis (CVT)
- thrombophilia
- headache
- native and contrast-enhanced Head Computed Tomography (CT)
- Magnetic Resonance Imaging (MRI) of the Head
1. Epidemiology
No epidemiologic studies of cerebral venous thrombosis (CVT) own the needed criteria for a good quality epidemiologic stroke study, due to multiple factors, including the wide clinical spectrum of CVT, frequently with subacute onset [1][2][3].
CVT represents only less than 1% of all strokes [3], its prevalence is higher than previously noted, due to an increased awareness of this type of disease among clinicians, and to improved and more accessible imaging methods, including Magnetic Resonance Imaging (MRI)/MR Venography (MRV), for the examination of patients with unclear neurological clinical aspects, such as headache and seizures [1][2][3][4][5].
Annual incidence is noted to be between 0.22 to 1.57 per 100,000 citizens [2] and is sex-independent in children and the elderly [1][6]. CVT is more frequent in children (especially in neonates) than in adults [1][6]. In adults, CVT is observed in patients who are younger on average than those with arterial types of stroke [1]. Thus, in the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) cohort, the median age was 37 years [6], and only 8% of the patients were older than 65 [7]. Compared with men, women were significantly younger (median age of 34 years, vs. 42 years for men) [4]. In adult cases of the ISCVT cohort, CVT presented a female predominance (3:1) [8], which was higher between 31 to 50 years because of augmented risk attributed to a prothrombotic condition [1][6].
2. Etiology-Risk Factors
The most frequent risk factors detected in elder people with CVT are thrombophilia, neoplasms, and hematologic disorders [7][9].
In the Canadian pediatric ischemic stroke registry, a risk factor was detected in 98% of the children. Thrombophilia was found in 41% of all cases. In infants older than four weeks of age and children head and neck diseases, especially infections and chronic systemic diseases (e.g., connective tissue diseases, hematologic disorders, or malignancy) were frequent [10].
Different factors can determine or predispose adults to develop CVT (in 85% of cases at least one risk factor can be found), and in about half of CVT cases, they present multiple risk factors. For this reason, the detection of a cause or a risk factor should not stop a search for others [6]. The most common risk factors are represented by: genetic thrombophilia, oral contraceptives (OC), pregnancy, puerperium, malignancy, and infections [3][6]. Thus, a prothrombotic condition was detected in one-third of all cases of the ISCVT cohort, and a genetic thrombophilia was reported in 22% of all cases [6].
3. Pathophysiology
The main two pathophysiological mechanisms implied in CVT are diminution of CSF absorption, and increase of venular and capillary pressure [11][12][13][14] (Figure 1).
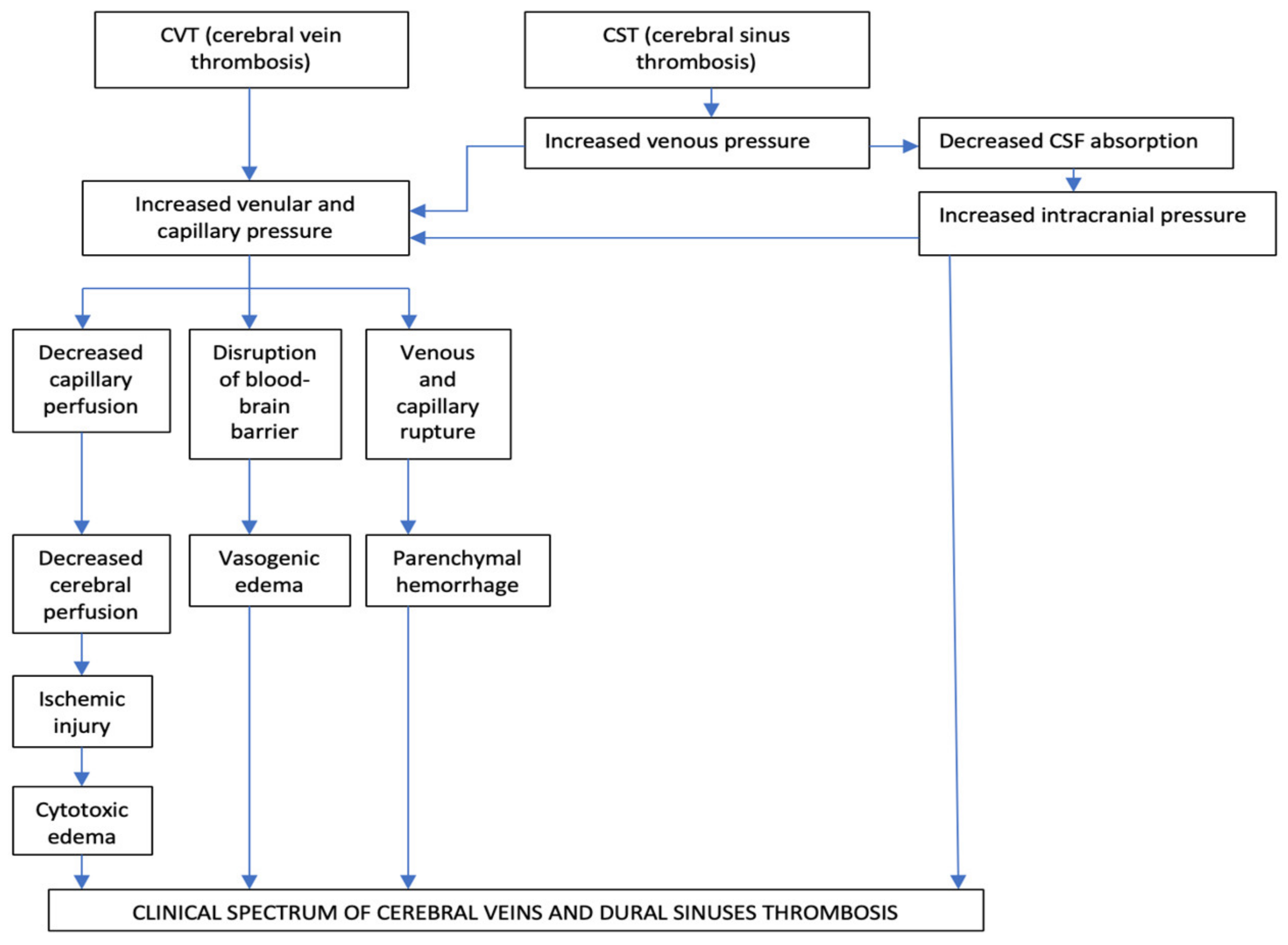
Figure 1. Pathophysiology of cerebral veins and dural sinuses thrombosis.
The Occlusion of the Intracranial Dural Sinuses May Determine a Diminution of CSF Absorption
The normal absorption of CSF is produced through arachnoid granulations, especially at the level of the SSS and LS. In the particular situation of dural sinuses occlusion, especially of the SSS and LS, appears a raise of the cerebral venous pressure, with subsequent diminution of CSF absorption which, consecutively, increases the intracranial pressure. This pathologic process produces a rise in venular and capillary hypertension and generates vasogenic and cytotoxic edema and cerebral hemorrhage [11][12][13][14].
The second mechanism is represented by the progressive increase of venular and capillary pressure.
This mechanism is the result of the thrombosis of dural sinuses and cerebral veins [11][12][13][14].
In the initial stages of venous obstruction, a diminished but still efficient perfusion of the correspondent brain tissue might be possible, due to the collateral venous circulation, which produces a significant degree of compensation, with consecutive neutralization of the pathological pressure modifications. For this reason, the corresponding areas of the brain can be functionally and metabolically affected, but not irreversibly injured [11][12][13][14]. As loco-regional venous pressure continues to increase, in the context of an ineffective collateral venous circulation, a rise of the thrombosis within cortical venous tributaries will reduce the cerebral perfusion pressure even more. Consequently, it will appear damage to the blood-brain barrier producing vasogenic edema, local ischemic lesions, cytotoxic edema, and venous and capillary lesions with consecutively parenchymal hemorrhage and, rarely, subarachnoid hemorrhages [11][12][13][14].
The diminution of the venous drainage consecutive to CVT determines raised venous pressure, with the backup of the fluid into the brain, producing vasogenic edema. This type of edema is situated within the extracellular compartment of the encephalic white matter/inside the glial cells, under the control of the hydrostatic pressure (augmented blood pressure and local blood flow) and osmotic gradients. Usually, the vasogenic edema does not produce neuronal lesions, because the fluid in excess in the extracellular space can, frequently, be removed [11][12][13][14].
Cytotoxic edema is produced by energy failure with a displacement of ions and water across the cell membranes into neurons. The intracellular edema, caused by ischemia, determines a great volume of dead or dying brain neurons with a bad prognosis [11][12][13][14].
In cerebral venous infarcts, the vasogenic edema represents the majority in comparison with the cytotoxic edema; these pathological aspects identified by diffusion-weighted imaging (DWI) confirm that the venous infarcts differ from arterial ones and present a significantly better recovery [12][13][14][15].
Brain edema and associated augmented intracranial pressure produce headache, vomiting, and diminished consciousness, but the most severe complications are represented by the pressure differences and the potential risk for brain herniation, which can determine death due to probable pressure-related lesions to neighboring areas [15].
The growth of the venous and capillary pressures produces vessel lesions and erythrocytes diapedesis due to disruptions of the blood-brain barrier both resulting in cerebral hemorrhage. The neuronal lesions determined by the cerebral hemorrhage induced by CVT are often milder than the damages induced by arterial infarcts [11][12][13][14].
Histological exam in CVT cases notes dilated cerebral veins, brain edema with flattened gyri, diminished sulci, compressed small ventricles, and ischemic neuronal lesions. The thrombus inside the cerebral veins is similar to other venous thrombi (when it is fresh, it presents a rich content in red blood cells and fibrin and a poor content in platelets; and, when it is chronic, it is replaced by fibrous tissue, frequently with recanalization) [15].
4. Clinical Diagnosis
The clinical spectrum and outcome of CVT are related to different factors: location and number of thrombosed sinuses and cerebral veins, as well as the presence of functional collateral pathways, absence or presence of parenchymal lesions (cytotoxic or vasogenic edema, hemorrhage), gender, age, etiology, and interval from onset to admission to hospital [1][16].
The clinical presentation of CVT can be polymorphous, and misleading. In the majority of cases (50–80% of patients), the onset is subacute [15][17].
4.1. Clinical Syndromes
In the majority of adult CVT cases, four major clinical syndromes have been noted in combination or isolation: Isolated intracranial hypertension, focal neurological deficits, seizures, and encephalopathy [15][18].
A minority of adult CVT patients develop a cavernous sinus thrombosis with a distinctive clinical picture: painful ophthalmoplegia. Collet-Siquard syndrome (consisting of multiple low cranial nerves palsies) represents a clinical syndrome of IJVs, posterior fossa veins, or LS thrombosis [15][18].
Rare adult CVT cases with unusual clinical aspects were also reported: subarachnoid hemorrhage, transient ischemic attacks, or psychiatric symptoms, mimicking a postpartum psychosis [15][18].
In neonates, CVT has a nonspecific clinical presentation with seizures, tetraparesis, and encephalopathy [10]. In older children, the clinical spectrum is more similar to the adult clinical aspects, with headache and paresis [19]. In elderly patients, symptoms of encephalopathy are more common than in adults, whereas isolated intracranial hypertension is less common [15][18].
Isolated Intracranial Hypertension
It is the most frequent clinical syndrome observed in CVT (40% of cases) [1]. It consists of headaches, associated with vomiting, papilledema, visual complaints, and sixth nerve palsy [20]. It is more common in patients with a chronic onset than in those cases that present acutely [21].
Headache is the most common symptom of CVT (about 90% of cases in the ISCVT cohort). It is usually the initial one, and can develop isolated, or can precede other symptoms or signs. Headache is more frequent in women and younger patients than in men or older patients [4][6][17][19]. The characteristics of headaches are polymorphic. It may be localized or diffused [20]. Frequently, headache is severe augmenting during the night and may worsen with Valsalva maneuvers or position changes (when the patient is lying down) [18][22].
However, its characteristics can be misleading, sometimes being initially diagnosed as a migraine with aura [23]. In a few cases, it occurs like a thunderclap headache (mimicking a subarachnoid hemorrhage) [24]. Some of the risk factors associated with CVT (such as meningitis, epidural or brain abscesses, meningiomas, dural arteriovenous fistulas, and different vasculitis) also clinically manifest as a headache. CVT must be suspected as a possible explanation of persisting headache after lumbar puncture because this maneuver can rarely precipitate a CVT [15]. Headache is noted more frequently in patients with CVT than in cases with cerebral arterial infarcts [1][15].
Papilledema is observed on funduscopy in 25–40% of CVT cases, especially in those with chronic onset or delayed clinical presentation. It can produce transient loss of vision (associated with intense headache), and if prolonged, optic atrophy and consecutive peripheral blindness [1][3].
Focal Neurological Deficits
Paresis, sometimes bilateral, is the most frequent focal neurological deficit associated with CVT (in the ISCVT cohort was noted in 37% of cases) [6][15].
Other signs are less common: fluent aphasia (which is observed in left transverse sinus thrombosis associated with a posterior left temporal lesion), central sensory deficits, hemianopia, and ataxia (usually observed in posterior dural sinuses occlusion) [6]. Mixed transcortical aphasia is noted in left thalamus lesions due to deep cerebral vein thrombosis [25].
Seizures
Focal or generalized seizures, even status epilepticus, are more frequently noted during the evolution of CVT (in the ICSVT cohort in 40% of cases) [6] than in arterial strokes [26][27][28]. Seizures appear during the onset of CVT in about 12–15% of cases [26][27]. A higher incidence has been observed in peripartum (76%) [27] and neonates (44%) [28].
Early seizures are noted more frequently in cases with supratentorial parenchymal brain lesions (especially disposed anterior to the sulcus of Rolando), thrombosis of the SSS and cortical veins and in those patients who present motor deficits [26][27][28].
Encephalopathy
Subacute/chronic encephalopathy, presenting altered mental status with cognitive dysfunction (including delirium, apathy, and dysexecutive syndrome), and diminished level of consciousness (between drowsiness and deep coma) is frequently associated with multifocal neurological deficits and is observed especially in elderly patients or neonates with CVT [7][29]. Usually, the diminution of the level of consciousness is reversible; however, coma at onset represents the main predictor of a poor outcome [15][18].
4.2. Topographic Clinical Diagnosis
Due to frequent concomitant multiple cerebral veins and dural sinuses thrombosis (more than two-thirds of cases), the existence of multiple anatomic variants of some cerebral veins and dural sinuses, and action of venous collateral circulation, the topographic clinical diagnosis of CVT is not so well-defined like in arterial occlusion and frequent is misleading [6][15][30].
However, isolated thrombosis of the different dural sinuses and cerebral veins produces the following clinical aspects (Figure 2).
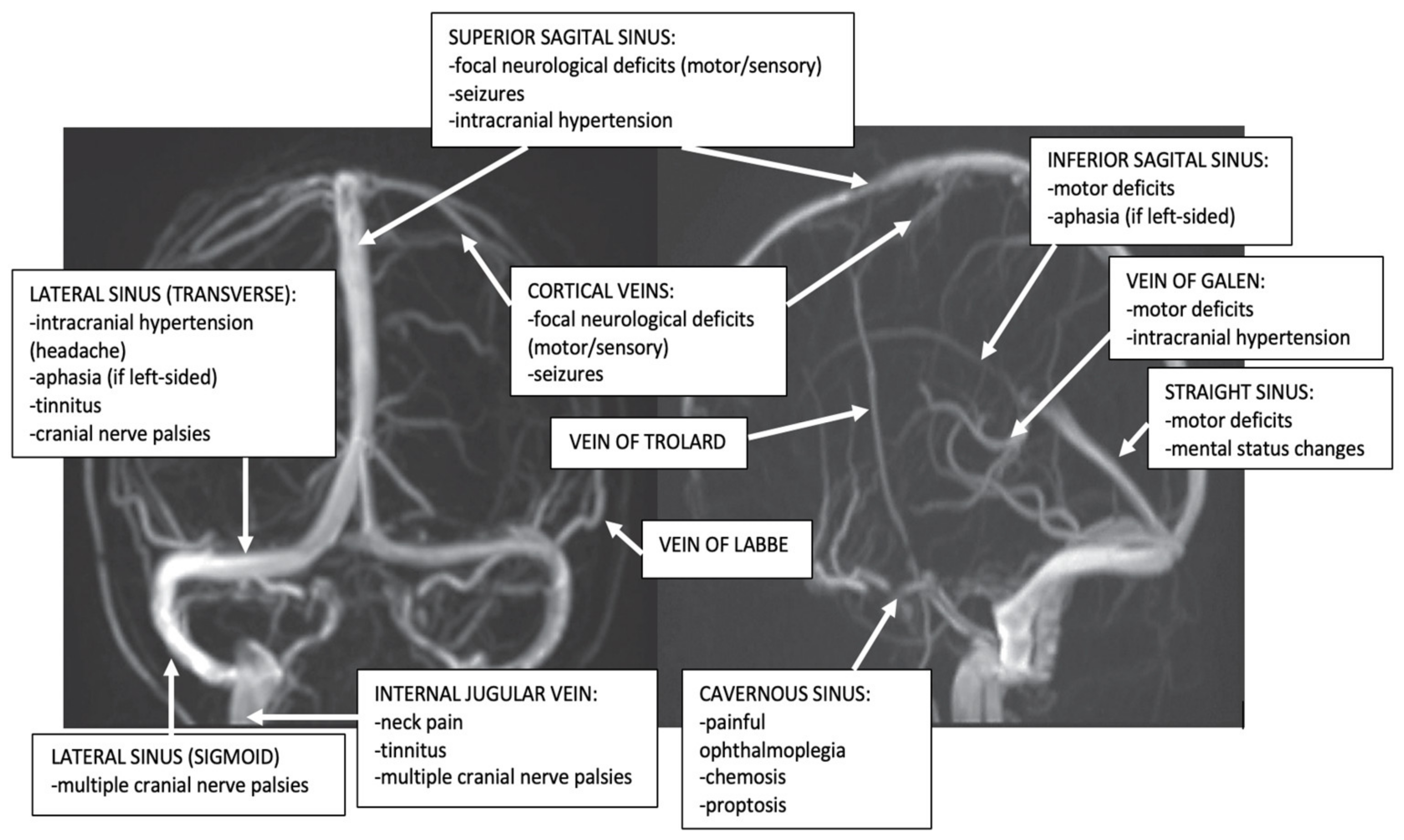
Figure 2. Dural sinuses and cerebral veins anatomy and major clinical syndromes according to the topography of CVT (archive of the First Department of Neurology, Clinical Emergency County Hospital, Timisoara, Romania).
Superior Sagittal Sinus (SSS) Thrombosis
It represents the most frequent dural sinuses occlusion, especially during the puerperium (62–80% in association, and 30% in isolated thrombosis) [1][3][15]. The common clinical presentation is that of an isolated intracranial hypertension syndrome. The clinical aspects may vary depending on the concomitant occlusion of other dural sinuses, especially LSs cerebral, or tributaries cerebral veins. Bilateral motor/sensory signs (especially in the legs) and psychiatric symptoms (prefrontal syndrome) may also appear due to bilateral frontoparietal hemispheric lesions produced by the progression of the SSS thrombosis to tributaries bilateral cortical veins [1][3][15].
Lateral Sinus (LS) Thrombosis
LS thrombosis may present different clinical aspects. Cases with isolated LS thrombosis develop an isolated headache or frequently intracranial hypertension (pseudotumor) syndrome. Less often, these patients may also present with focal neurological deficits due to hemispheric lesions produced by the progression of the LS thrombosis to tributaries cortical veins.
In contrast to SSS thrombosis, the infectious etiology is much more common in LS thrombosis. Different localized pyogenic infections of the ears (otitis), mastoid air cells (mastoiditis), and the paranasal sinuses (sinusitis) can determine septic LS thrombosis: “otitic hydrocephalus” [15][30]. The clinical signs are relatively characteristic: fever, headache, neck pain, neck tenderness, nausea and vomiting, vertigo, diplopia produced by sixth nerve palsy, and temporal and retro-orbital pain due to symptomatic trigeminal neuralgia [3][15][30].
Since the left LS is often hypoplasic, the pseudotumor syndrome appears especially after right LS thrombosis. In such cases, a bilateral venous drainage impairment may be noted, affecting the inferior portions of both temporal lobes and cerebellum, with subsequent temporal lobe and cerebellar signs [15][18][30]. Fluent Wernicke aphasia is usually observed in left transverse sinus thrombosis associated with adjacent cerebral veins occlusion (40%) and can be associated with right hemianopia or superior quadrantanopia. Right temporal lobe lesions produce left hemianopia. Nystagmus and gait ataxia represent the markers of cerebellar affection [6][15][30].
Unusually, an isolated left LS thrombosis presents a misleading isolated headache (migraine-like). In such cases, the thrombosis is not due to an otitis, but a thrombophilia [11][30][31]. This is why screening for LS thrombosis (and other CVT) has to be done in young women with a recent headache even if this symptom is isolated and is not associated with otitis or mastoiditis [30][31]. LS thrombosis may present also as isolated pulsating tinnitus [32].
Cavernous Sinus Thrombosis
It is rare and usually has an infection etiology (pyogenic infections of the face, or the paranasal sinuses) [33][34].
In patients with classic acute unilateral septic cavernous sinus thrombosis, they present a typical clinical picture, with painful complete or partial ophthalmoplegia associated with chemosis, proptosis, and conjunctival edema. Frequently, a papilledema associated with hemorrhages of the retina can be observed. In the absence of an immediate diagnosis and treatment, it becomes bilateral via inter-cavernous sinuses. When the thrombosis progresses to other dural sinuses and cortical veins, seizures and paresis may associate [33][34].
In a minority of cases (head trauma, surgery on intracranial or facial structures, thrombophilia, and thrombosis of dural arteriovenous fistulas) an aseptic cavernous sinus thrombosis may be observed with an isolated abducens nerve palsy and mild proptosis [33].
Thrombosis of the Superior and Inferior Petrosal Sinuses
In the majority of cases, it represents a sequela of cavernous or sigmoid occlusion. The thrombosis of the superior petrosal sinus clinically manifests as a trigeminal palsy, while the occlusion of the inferior one occurs as an abducens palsy [15][18].
Cortical Veins Thrombosis
Isolated thrombosis of cortical veins without associated dural sinus thrombosis is considered a rare disease (2%), but it is probably underdiagnosed, due to difficulties to detect it using the traditional MRI sequences and MRV [35]. Occlusion appears especially at the levels of the superior cortical veins, producing seizures associated with motor/sensory deficits [35].
Thrombosis of the Deep Cerebral Venous System
Frequently associated with the thrombosis of the SS, it is a rare disease that occurs more often during childhood (especially in neonates). The clinical presentation is severe, with encephalopathy, and bilateral motor deficits [36][37].
In adults, a more limited thrombosis of the deep cerebral veins, without associated SS thrombosis, can produce relatively mild clinical aspects, such as headache, nausea and vomiting, gait ataxia, hemiparesis (that may be bilateral or alternating), neuropsychological symptoms, and mild disturbances of consciousness [36][37]. In rare situations, benign cases of thrombosis of the deep cerebral veins were noted with mixed transcortical aphasia [25].
Thrombosis of the Posterior Fossa Veins
Isolated venous infarctions in the posterior fossa are rare because the posterior fossa owns an efficient collateral venous circulation. However, this disease represents the main differential diagnosis in cases with concomitant risk factors for CVT, associated with some clinical aspects (intracranial hypertension syndrome, cerebellar-vestibular syndrome), and atypical aspects on brain CT (bilateral hemispheric and vermian cerebellar infarcts, irregular cerebellar hemorrhages) [38][39].
Internal Jugular Vein (IJV) Thrombosis
IJV thrombosis represents especially a progression of the sigmoid sinus thrombosis or may be produced by cannulation for long-term IJV access. It can be asymptomatic or its clinical picture consists of symptoms and signs of local infection (such as pain and swelling of the mastoid area, a painful and tender thrombosed IJV). The thrombophlebitis of the IJV can be a consequence of the syndrome of tonsillopharyngitis (Lemierre’s syndrome). A jugular foramen syndrome (consisting of unilateral pulsatile tinnitus [32] or multiple cranial nerve palsies VIII-XII) occurs if the infection affects the skull base [40].
Emissary Veins (EV) Thrombosis
The EVs (e.g., petrosquamosal sinus (PSS)), are vestigial valve-less veins, which connect the intracranial dural venous sinuses and the extracranial venous system. Posterior fossa EVs pass through corresponding cranial apertures and ensure (together with the IJV) an additional extracranial venous drainage of the veins of the posterior fossa. In healthy people, EVs are small and have no clinical significance. In pathological cases (associated with high-flow arteriovenous malformations, IJV aplasia or IJV thrombosis, or even LS thrombosis) they are large with clinical significance (different craniofacial syndromes and pulsatile tinnitus) [32][39].
This entry is adapted from the peer-reviewed paper 10.3390/life12050717
References
- Ferro, J.M.; Canhão, P. Cerebral Venous Thrombosis: Etiology, Clinical Features, and Diagnosis. Available online: https://www.uptodate.com/contents/cerebral-venous-thrombosis-etiology-clinical-features-and-diagnosis (accessed on 15 October 2021).
- Coutinho, J.M.; Zurbier, S.M.; Aramideh, M.; Stam, J. The Incidence of Cerebral Venous Thrombosis A Cross-Sectional Study. Stroke 2012, 43, 3375–3377.
- Saposnik, G.; Barinagarrementeria, F.; Brown, R.D.; Bushnell, C.D.; Cucchiara, B.; Cushman, M.; de Veber, G.; Ferro, J.M.; Tsai, F.Y.; on behalf of the American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 1158–1192.
- Coutinho, J.M.; Ferro, J.M.; Canhão, P.; Barinagarrementeria, F.; Bousser, M.G.; Stam, J. Cerebral Venous and Sinus Thrombosis in Women. Stroke 2009, 40, 2356–2361.
- Devasagayam, S.; Wyatt, B.; Leyden, J.; Kleinig, T. Cerebral Venous Sinus Thrombosis Incidence Is Higher Than Previously Thought: A Retrospective Population-Based Study. Stroke 2016, 47, 2180.
- Ferro, J.M.; Canhao, P.; Stam, J.; Bousser, M.G.; Barinagarrementeria, F. Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 2004, 35, 664–670.
- Ferro, J.M.; Canhão, P.; Bousser, M.-G.; Stam, J.; Barinagarrementeria, F. Cerebral vein and dural sinus thrombosis in elderly patients. Stroke 2005, 36, 1927.
- Satyarthee, G.D.; Moscote-Salazar, L.R.; Agrawal, A. Persistent enlarged occipital sinus with absent unilateral transverse sinus. J. Neurosci. Rural. Pract. 2019, 10, 519–521.
- Zuurbier, S.M.; Hiltunen, S.; Lindgren, E.; Silvis, S.M.; Jood, K.; Devasagayam, S.; Kleinig, T.; Silver, F.; Mandell, D.M.; Putaala, J.; et al. Cerebral Venous Thrombosis in Older Patients. Stroke 2018, 49, 197.
- DeVeber, G.; Andrew, M.; Adams, C.; Bjornson, B.; Booth, F.; Buckley, D.J.; Camfield, C.S.; David, M.; Humphreys, P.; Langevin, P.; et al. Cerebral sinovenous thrombosis in children. N. Engl. J. Med. 2001, 345, 417.
- Piazza, G. Cerebral Venous Thrombosis. Circulation 2012, 125, 1704–1709.
- Schaller, B.; Graf, R. Cerebral Venous Infarction-The Pathophysiological Concept. Cerebrovasc. Dis. 2004, 18, 179–188.
- Gotoh, M.; Ohmoto, T.; Kuyama, H. Experimental study of venous circulatory disturbance by dural sinus occlusion. Acta Neurochir. 1993, 124, 120.
- Lövblad, K.O.; Bassetti, C.; Schneider, J.; Guzman, R.; El-Koussy, M.; Remonda, L.; Schroth, G.S.O. Diffusion-weighted mr in cerebral venous thrombosis. Cereb. Dis. 2001, 11, 169.
- Bousser, M.G.; Barnett, H.J.M. Chapter Twelve: Cerebral Venous Thrombosis. In Stroke (Pathophysiology, Diagnosis, and Management), 4th ed.; Mohr, J.P., Choi, D.W., Grotta, J.C., Weir, B., Wolf, P.A., Eds.; Churchill Livingstone: London, UK, 2004; pp. 301–325.
- Dmytriw, A.A.; Song, J.S.A.; Yu, E.; Poon, C.S. Cerebral venous thrombosis: State of the art diagnosis and management. Neuroradiology 2018, 60, 669–685.
- Bousser, M.G.; Chiras, J.; Bories, J.; Castaigne, P. Cerebral venous thrombosis-a review of 38 cases. Stroke 1985, 16, 199.
- Stam, J. Thrombosis of the cerebral veins and sinuses. N. Engl. J. Med. 2005, 352, 1791–1798.
- Ichord, R.N.; Benedict, S.L.; Chan, A.K.C.; Kirkham, F.; Nowakgottl, U. Paediatric cerebral sinovenous thrombosis: Findings of the International Paediatric Stroke Study. Arch. Dis. Child. 2015, 100, 174.
- Biousse, V.; Ameri, A.; Bousser, M.G. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology 1999, 53, 1537.
- Lopes, M.G.; Ferro, J.; Pontes, C. Henriques I for the Venoport Investigators. Headache and cerebral venous thrombosis. Cephalalgia 2000, 20, 292.
- Bousser, M.G.; Crassard, I. Cerebral venous thrombosis, pregnancy and oral contraceptives. Thromb. Res. 2012, 130, S19–S22.
- Cumurciuc, R.; Crassard, I.; Sarov, M.; Valade, D.; Bousser, M.G. Headache as the only neurological sign of cerebral venous thrombosis: A series of 17 cases. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1084.
- de Bruijn, S.F.; Stam, J.; Kappelle, L.J. Thunderclap headache as first symptom of cerebral venous sinus thrombosis. CVST Study Group. Lancet 1996, 348, 1623.
- Jianu, D.C.; Jianu, S.N.; Dan, T.F.; Iacob, N.; Munteanu, G.; Motoc, A.G.M.; Baloi, A.; Hodorogea, D.; Axelerad, A.D.; Ples, H.; et al. Diagnosis and Management of Mixed Transcortical Aphasia Due to Multiple Predisposing Factors, including Postpartum and Severe Inherited Thrombophilia, Affecting Multiple Cerebral Venous and Dural Sinus Thrombosis: Case Report and Literature Review. Diagnostics 2021, 11, 1425.
- Ferro, J.M.; Canhão, P.; Bousser, M.G.; Stam, J.; Barinagarrementeria, F. Early Seizures in Cerebral Vein and Dural Sinus Thrombosis Risk Factors and Role of Antiepileptics. Stroke 2008, 39, 1152–1158.
- Ferro, J.M.; Correia, M.; Rosas, M.J.; Pinto, A.N.; Neves, G.; The Cerebral Venous Thrombosis Portuguese Collaborative Study Group . Seizures in cerebral vein and dural sinus thrombosis. Cereb. Dis. 2003, 15, 78–83.
- Lancon, J.; Killough, K.; Tibbs, R.; Lewis, A.; Parent, A. Spontaneous dural sinus thrombosis in children. Pediatr. Neurosurg. 1999, 3, 23.
- Ferro, J.M.; Correia, M.; Pontes, C.; Baptista, M.V.; Pita, F. Cerebral vein and dural sinus thrombosis in Portugal: 1980–1998. Cerebrovasc. Dis. 2001, 11, 177.
- Damak, M.; Crassard, I.; Wolff, V.; Bousser, M.G. Isolated Lateral Sinus Thrombosis A Series of 62 Patients. Stroke 2009, 40, 476–481.
- Jianu, D.C.; Jianu, S.N.; Motoc, A.G.M.; Poenaru, M.; Petrica, L.; Vlad, A.; Ursoniu, S.; Gogu, A.E.; Dan, T.F. Diagnosis and management of a young woman with acute isolated lateral sinus thrombosis. Rom. J. Morphol. Embryol. 2017, 58, 1515–1518.
- Waldvogel, D.; Mattle, H.P.; Sturzenegger, M.; Schroth, G. Pulsatile tinnitus—A review of 84 patients. J. Neurol. 1998, 245, 137.
- Sakaida, H.; Kobayashi, M.; Ito, A.; Takeuchi, K. Cavernous sinus thrombosis: Linking a swollen red eye and headache. Lancet 2014, 384, 928.
- Ebright, J.R.; Pace, M.T.; Niazi, A.F. Septic thrombosis of the cavernous sinuses. Arch. Intern. Med. 2001, 161, 2671.
- Jacobs, K.; Moulin, T.; Bogousslavsky, J.; Woimant, F.; Dehaene, I.; Tatu, L.; Besson, G.; Assouline, E.; Casselman, J. The stroke syndrome of cortical vein thrombosis. Neurology 1996, 47, 376.
- Lacour, J.C.; Ducrocq, X.; Anxionnat, R.; Taillandier, L.; Auque, J.; Weber, M. Thrombosis of deep cerebral veins in form adults: Clinical features and diagnostic approach. Rev. Neurol. 2000, 156, 851.
- van den Bergh, W.M.; van der Schaaf, I.; van Gijn, J. The spectrum of presentations of venous infarction caused by deep cerebral vein thrombosis. Neurology 2005, 65, 192.
- Pekçevik, Y.; Pekçevik, R. Why should we report posterior fossa emissary veins? Diagn. Interv. Radiol. 2014, 20, 78–81.
- Jianu, D.C.; Jianu, S.N.; Dan, T.F.; Motoc, A.G.M.; Poenaru, M. Pulsatile tinnitus caused by a dilated left petrosquamosal sinus. Rom. J. Morphol. Embryol. 2016, 57, 319–322. Available online: www.rjme.ro (accessed on 16 May 2021).
- Kuehnen, J.; Schwartz, A.; Neff, W.; Hennerici, M. Cranial nerve syndrome in thrombosis of the transverse/sigmoid sinuses. Brain 1998, 121, 381.
This entry is offline, you can click here to edit this entry!
