The rhizosphere serves as a hotspot for diverse microbial activity. It is an intricate ecosystem comprising nutrient-rich soil that surrounds the plant roots, which provides a pool for plant–microbe communication. The term “Rhizomicrobiome” is defined as a microbial community that is present in the rhizosphere. A variety of microorganisms reside within the rhizosphere, including bacteria, fungi, nematodes, protists, and invertebrates. Most of the microbiome studies within the context of rhizosphere signaling have been focused on the bacteria and fungi that make up the major portion of the rhizosphere microbiome.
- rhizosphere
- microbial metabolites
- plant–microbe signaling
- holobiont
- plant-microbe communications
- rhizomicrobiome
- quorum-sensing
1. Rhizosphere: A Pool of Plant–Microbe Signaling
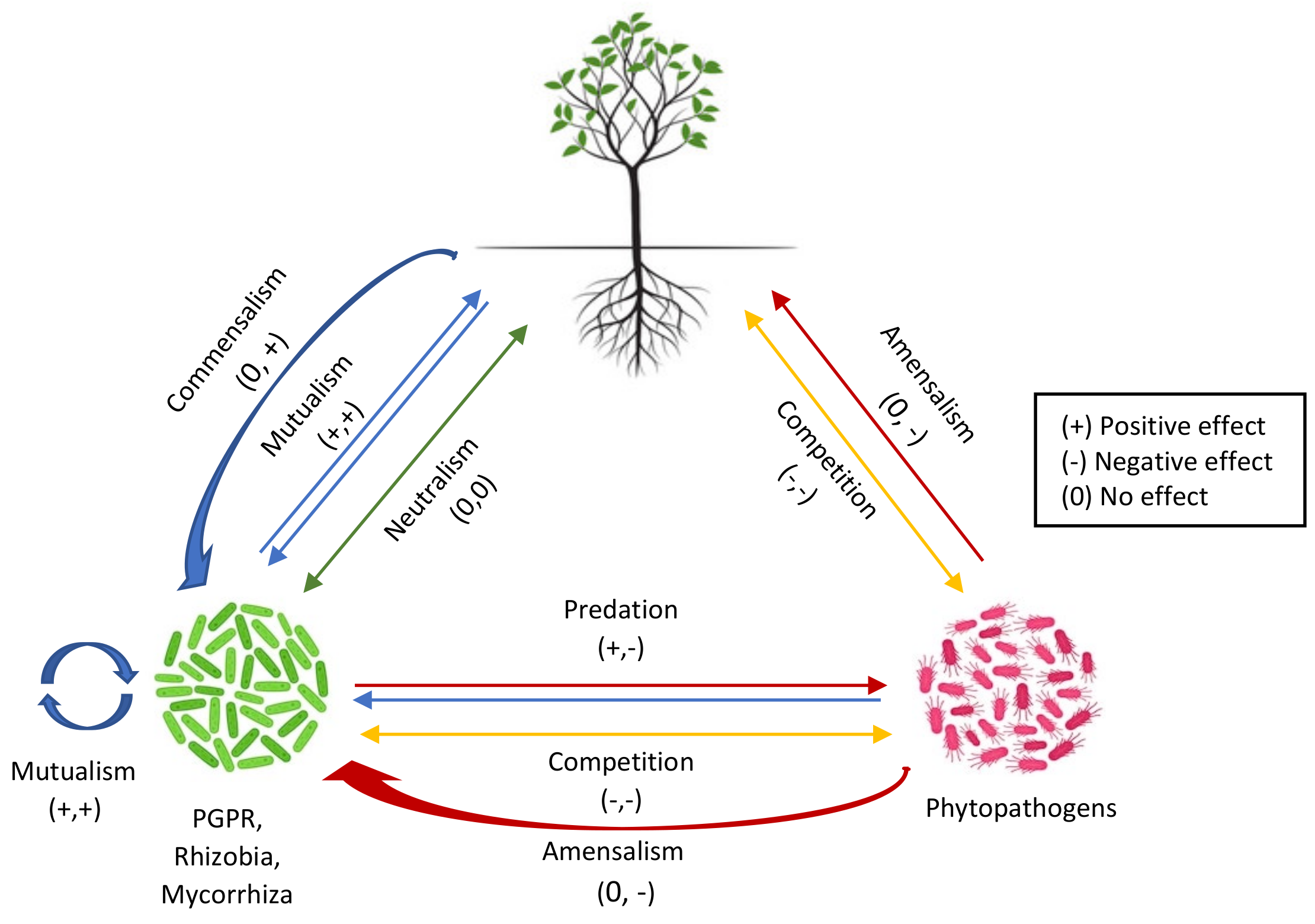
2. Inter- or Intraspecies Signaling among Microorganisms
| Type of Microbe | Rhizo-Microorganisms | Quorum Sensing Molecules | Type of Communication | Reference |
|---|---|---|---|---|
| Gram-positive bacteria | Bacillus subtilis | ComX pheromone | Inter- and intraspecies | [31] |
| Streptomyces spp. | Gamma-butyrolactones (A-factor) and methylenomycin furans (MMF1) | Interspecies | [32] | |
| Staphylococcus aureus | Circular oligopeptide | Interspecies | [29] | |
| Stenotrophomonas chelatiphaga | DSF (diffuse signal factor) | Interkingdom (poplar plant) | [33] | |
| Bacillus velezensis | AI-2 synthetase (2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (THMF)) | Interkingdom (maize plant) | [34] | |
| Gram-negative bacteria | Burkholderia sp. | N-3-oxo-hexanoyl-homoserine lactone (3OC6-HSL) | Interkingdom (wheat and arabidopsis plant) | [35] |
| Serratia glossinae | N-hexanoyl-L-homoserine lactone (m/z 200) and N-octanoyl-L-homoserine lactone (m/z 228) | Interkingdom (rice plant) |
[36] | |
| Serratia plymuthica | N-butanoyl-HSL, N-hexanoyl-HSL, and N-3-oxo-hexanoyl-HSL (OHHL) | Interkingdom (oil seed rape) | [37] | |
| Burkholderia graminis M12 and B. graminis M14 | N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL or OC12-HSL (where “O” indicates an oxo substitution at the third carbon atom)) and 3-oxo-C14-HSL (OC14-HSL) | Interkingdom (tomato plant) | [38] | |
| Serratia marcescens | N-3-oxo-hexanoyl-homoserine lactone (3OC6-HSL) | Intraspecies and interkingdom (tobacco plant) | [39] | |
| Burkholderia sp. and Pseudomonas sp. | N-butyryl-homoserine lactone (C4-HSL) | Interkingdom (arabidopsis plant) | [40] | |
| Ochrobactrum sp. | 3O-C7-HSL and 3OH-C7-HSL | Interkingdom (bean plant) | [41] | |
| Stenotrophomonas maltophilia | DSF (diffuse signal factor) | Interkingdom (oil seed rape) | [42] | |
| Serratia glossinae | N-octanoyl-L-homoserine lactone and N-hexanoyl-L-homoserine lactone | Interkingdom (sesame plant) | [36] | |
| Fungi | Candida albicans | Tyrosol, γ-butyrolactone, and farnesol | Intraspecies | [43] |
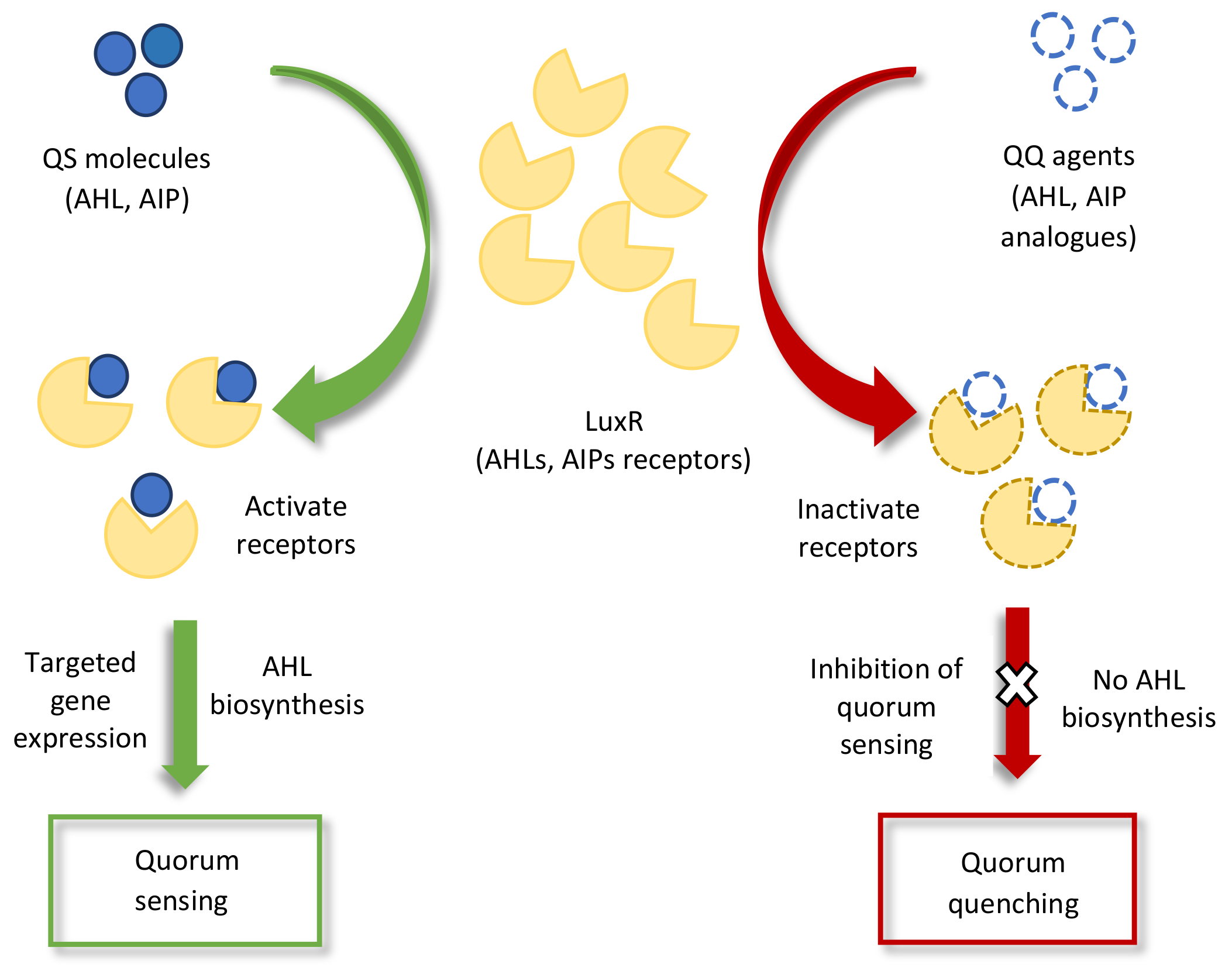
3. Interkingdom Signaling
3.1. Microbe–Plant Signaling
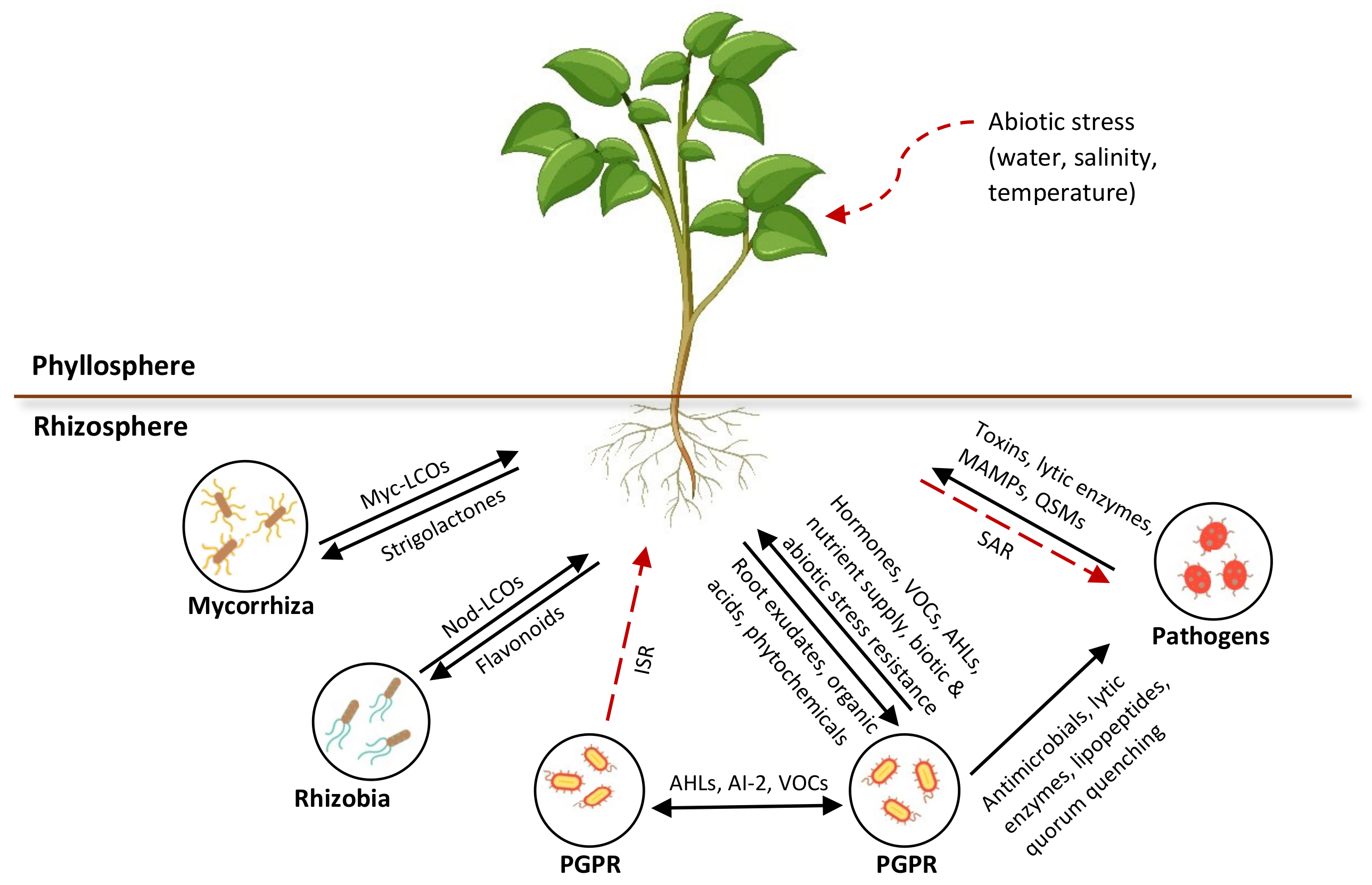
3.2. Plant–Microbe Signaling
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10050899
References
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O.; Ayangbenro, A. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166.
- Venturi, V.; Keel, C. Signaling in the rhizosphere. Trends Plant Sci. 2016, 21, 187–198.
- Pratama, A.A.; Terpstra, J.; de Oliveria, A.L.M.; Salles, J.F. The Role of Rhizosphere Bacteriophages in Plant Health. Trends Microbiol. 2020, 28, 709–718.
- Sharma, R.S.; Nayak, S.; Malhotra, S.; Karmakar, S.; Sharma, M.; Raiping, S.; Mishra, V. Rhizosphere provides a new paradigm on the prevalence of lysogeny in the environment. Soil Tillage Res. 2019, 195, 104368.
- Li, Y.; Sun, H.; Yang, W.; Chen, G.; Xu, H. Dynamics of Bacterial and Viral Communities in Paddy Soil with Irrigation and Urea Application. Viruses 2019, 11, 347.
- Simon, J.-C.; Marchesi, J.; Mougel, C.; Selosse, M.-A. Host-Microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5.
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41.
- Mommer, L.; Kirkegaard, J.; van Ruijven, J. Root–Root Interactions: Towards a rhizosphere framework. Trends Plant Sci. 2016, 21, 209–217.
- Rosier, A.; Bishnoi, U.; Lakshmanan, V.; Sherrier, D.J.; Bais, H.P. A perspective on inter-kingdom signaling in plant–beneficial microbe interactions. Plant Mol. Biol. 2016, 90, 537–548.
- Hein, J.W.; Wolfe, G.V.; Blee, K.A. Comparison of Rhizosphere Bacterial Communities in Arabidopsis thaliana mutants for systemic Acquired Resistance. Microb. Ecol. 2008, 55, 333–343.
- Schlaeppi, K.; Bulgarelli, D. The Plant Microbiome at Work. Mol. Plant-Microbe Interact. 2015, 28, 212–217.
- Lareen, A.; Burton, F.; Schäfer, P. Plant root-microbe communication in shaping root microbiomes. Plant Mol. Biol. 2016, 90, 575–587.
- Yang, C.-H.; Crowley, D.E. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl. Environ. Microbiol. 2000, 66, 345–351.
- Malusà, E.; Pinzari, F.; Canfora, L. Efficacy of biofertilizers: Challenges to improve crop production. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 17–40.
- Jones, P.; Garcia, B.; Furches, A.; Tuskan, G.A.; Jacobson, D. Plant host-associated mechanisms for microbial selection. Front. Plant Sci. 2019, 10, 862.
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663.
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369.
- Hong, K.W.; Koh, C.L.; Sam, C.-K.; Yin, W.-F.; Chan, K.-G. Quorum Quenching Revisited—From Signal Decays to Signalling Confusion. Sensors 2012, 12, 4661–4696.
- Helman, Y.; Chernin, L. Silencing the mob: Disrupting quorum sensing as a means to fight plant disease. Mol. Plant Pathol. 2015, 16, 316–329.
- Schikora, A.; Schenk, S.T.; Hartmann, A. Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl-homoserine lactone group. Plant Mol. Biol. 2016, 90, 605–612.
- Hassan, R.; Shaaban, M.I.; Bar, F.A.; El-Mahdy, A.; Shokralla, S. Quorum sensing inhibiting activity of Streptomyces coelicoflavus Isolated from Soil. Front. Microbiol. 2016, 7, 659.
- Chu, W. Role of the quorum-sensing system in biofilm formation and virulence of Aeromonas hydrophila. Afr. J. Microbiol. Res. 2011, 5, 5819–5825.
- Atkinson, S.; Williams, P. Quorum sensing and social networking in the microbial world. J. R. Soc. Interface 2009, 6, 959–978.
- Checcucci, A.; Marchetti, M. The Rhizosphere Talk Show: The Rhizobia on Stage. Front. Agron. 2020, 2, 591494.
- Vendeville, A.; Winzer, K.; Heurlier, K.; Tang, C.M.; Hardie, K. Making ‘sense’ of metabolism: Autoinducer-2, LUXS and pathogenic bacteria. Nat. Rev. Genet. 2005, 3, 383–396.
- Winzer, K. Bacterial cell-to-cell communication: Sorry, can’t talk now—Gone to lunch! Curr. Opin. Microbiol. 2002, 5, 216–222.
- Zhang, W.; Li, C. Exploiting Quorum Sensing Interfering Strategies in Gram-negative bacteria for the enhancement of environmental applications. Front. Microbiol. 2016, 6, 1535.
- Ferluga, S.; Steindler, L.; Venturi, V. N-acyl homoserine lactone quorum sensing in Gram-negative rhizobacteria. In Secondary Metabolites in Soil Ecology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 69–90.
- Borges, A.; Simões, M. Quorum Sensing Inhibition by marine bacteria. Mar. Drugs 2019, 17, 427.
- Sindhu, S.S.; Sehrawat, A.; Sharma, R.; Dahiya, A.; Khandelwal, A. Belowground microbial crosstalk and rhizosphere biology. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 695–752.
- Selvakumar, G.; Panneerselvam, P.; Ganeshamurthy, A.N. Bacterial mediated alleviation of abiotic stress in crops. In Bacteria in Agrobiology: Stress Management; Springer: Berlin/Heidelberg, Germany, 2012; pp. 205–224.
- Aigle, B.; Corre, C. Waking up Streptomyces Secondary Metabolism by Constitutive Expression of Activators or Genetic Disruption of Repressors. Methods Enzymol. 2012, 517, 343–366.
- Ulrich, K.; Kube, M.; Becker, R.; Schneck, V.; Ulrich, A. Genomic analysis of the endophytic Stenotrophomonas strain 169 reveals features related to plant-growth promotion and stress tolerance. Front. Microbiol. 2021, 12, 1542.
- Xiong, Q.; Liu, D.; Zhang, H.; Dong, X.; Zhang, G.; Liu, Y.; Zhang, R. Quorum sensing signal autoinducer-2 promotes root colonization of Bacillus velezensis SQR9 by affecting biofilm formation and motility. Appl. Microbiol. Biotechnol. 2020, 104, 7177–7185.
- Zhao, Q.; Yang, X.-Y.; Li, Y.; Liu, F.; Cao, X.-Y.; Jia, Z.-H.; Song, S.-S. N-3-oxo-hexanoyl-homoserine lactone, a bacterial quorum sensing signal, enhances salt tolerance in Arabidopsis and wheat. Bot. Stud. 2020, 61, 8–12.
- Jung, B.K.; Khan, A.R.; Hong, S.-J.; Park, G.-S.; Park, Y.-J.; Kim, H.-J.; Jeon, H.-J.; Khan, M.A.; Waqas, M.; Lee, I.-J.; et al. Quorum sensing activity of the plant growth-promoting rhizobacterium Serratia glossinae GS2 isolated from the sesame (Sesamum indicum L.) rhizosphere. Ann. Microbiol. 2017, 67, 623–632.
- Liu, X.; Bimerew, M.; Ma, Y.; Müller, H.; Ovadis, M.; Eberl, L.; Berg, G.; Chernin, L. Quorum-Sensing signaling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain of Serratia plymuthica. FEMS Microbiol. Lett. 2007, 270, 299–305.
- Barriuso, J.; Solano, B.R.; Fray, R.G.; Cámara, M.; Hartmann, A.; Mañero, F.J.G. Transgenic tomato plants alter quorum sensing in plant growth-promoting rhizobacteria. Plant Biotechnol. J. 2008, 6, 442–452.
- Ryu, C.-M.; Choi, H.K.; Lee, C.-H.; Murphy, J.F.; Lee, J.-K.; Kloepper, J.W. Modulation of Quorum Sensing in Acyl-homoserine Lactone-Producing or -Degrading Tobacco Plants Leads to Alteration of Induced Systemic Resistance Elicited by the Rhizobacterium Serratia marcescens 90–166. Plant Pathol. J. 2013, 29, 182–192.
- Song, S.; Jia, Z.; Xu, J.; Zhang, Z.; Bian, Z. N-butyryl-homoserine lactone, a bacterial quorum-sensing signaling molecule, induces intracellular calcium elevation in Arabidopsis root cells. Biochem. Biophys. Res. Commun. 2011, 414, 355–360.
- Imran, A.; Saadalla, M.J.A.; Khan, S.-U.; Mirza, M.S.; Malik, K.A.; Hafeez, F.Y. Ochrobactrum sp. Pv2Z2 exhibits multiple traits of plant growth promotion, biodegradation and N-acyl-homoserine-lactone quorum sensing. Ann. Microbiol. 2014, 64, 1797–1806.
- Alavi, P.; Müller, H.; Cardinale, M.; Zachow, C.; Sanchez, M.B.; Martinez, J.L.; Berg, G. The DSF Quorum Sensing System Controls the Positive Influence of Stenotrophomonas maltophilia on Plants. PLoS ONE 2013, 8, e67103.
- De Sordi, L.; Mühlschlegel, F.A. Quorum sensing and fungal-bacterial interactions inCandida albicans: A communicative network regulating microbial coexistence and virulence. FEMS Yeast Res. 2009, 9, 990–999.
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478.
- Brescia, F.; Marchetti-Deschmann, M.; Musetti, R.; Perazzolli, M.; Pertot, I.; Puopolo, G. The rhizosphere signature on the cell motility, biofilm formation and secondary metabolite production of a plant-associated Lysobacter strain. Microbiol. Res. 2020, 234, 126424.
- Hartmann, A.; Schikora, A. Quorum Sensing of Bacteria and Trans-Kingdom Interactions of N-Acyl Homoserine Lactones with Eukaryotes. J. Chem. Ecol. 2012, 38, 704–713.
- Clinton, A.; Rumbaugh, K.P. Interspecies and Interkingdom Signaling via Quorum Signals. Isr. J. Chem. 2016, 56, 265–272.
- Bitas, V.; Kim, H.-S.; Bennett, J.W.; Kang, S. Sniffing on Microbes: Diverse Roles of Microbial Volatile Organic Compounds in Plant Health. Mol. Plant-Microbe Interact. 2013, 26, 835–843.
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M. Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci. 2015, 20, 206–211.
- Kai, M.; Effmert, U.; Piechulla, B. Bacterial-Plant-Interactions: Approaches to Unravel the Biological Function of Bacterial Volatiles in the Rhizosphere. Front. Microbiol. 2016, 7, 108.
- Tyc, O.; Zweers, H.; de Boer, W.; Garbeva, P. Volatiles in Inter-Specific Bacterial Interactions. Front. Microbiol. 2015, 6, 1412.
- Audrain, B.; Farag, M.A.; Ryu, C.-M.; Ghigo, J.-M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233.
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335.
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric Oxide Signaling in Pseudomonas aeruginosa Biofilms Mediates Phosphodiesterase Activity, Decreased Cyclic Di-GMP Levels, and Enhanced Dispersal. J. Bacteriol. 2009, 191, 7333–7342.
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome Survey and Characterization of Endophytic Bacteria Exhibiting a Beneficial Effect on Growth and Development of Poplar Trees. Appl. Environ. Microbiol. 2009, 75, 748–757.
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125.
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921.
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2018, 64, 574–587.
- Zhou, X.R.; Dai, L.; Xu, G.F.; Wang, H.S. A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Sci. Rep. 2021, 11, 7637.
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375.
- Vos, I.A.; Pieterse, C.; Van Wees, S. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55.
- Zamioudis, C.; Pieterse, C.M.J. Modulation of Host Immunity by Beneficial Microbes. Mol. Plant-Microbe Interact. 2012, 25, 139–150.
- Von Rad, U.; Klein, I.; Dobrev, P.I.; Kottova, J.; Zazimalova, E.; Fekete, A.; Hartmann, A.; Schmitt-Kopplin, P.; Durner, J. Response of Arabidopsis thaliana to N-hexanoyl-dl-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 2008, 229, 73–85.
- Kakkar, A.; Nizampatnam, N.R.; Kondreddy, A.; Pradhan, B.B.; Chatterjee, S. Xanthomonas campestriscell–cell signalling molecule DSF (diffusible signal factor) elicits innate immunity in plants and is suppressed by the exopolysaccharide xanthan. J. Exp. Bot. 2015, 66, 6697–6714.
- Corral-Lugo, A.; Daddaoua, A.; Ortega, A.; Espinosa-Urgel, M.; Krell, T. Rosmarinic acid is a homoserine lactone mimic produced by plants that activates a bacterial quorum-sensing regulator. Sci. Signal. 2016, 9, ra1.
- Hartmann, A.; Rothballer, M.; Hense, B.A.; Schröder, P. Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front. Plant Sci. 2014, 5, 131.
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a Sulfur-Rich Molecule from Garlic, Inhibits Genes Controlled by Quorum Sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325.
- Ganin, H.; Rayo, J.; Amara, N.; Levy, N.; Krief, P.; Meijler, M.M. Sulforaphane and erucin, natural isothiocyanates from broccoli, inhibit bacterial quorum sensing. MedChemComm 2013, 4, 175–179.
- Rudrappa, T.; Bais, H.P. Curcumin, a Known Phenolic from Curcuma longa, Attenuates the Virulence of Pseudomonas aeruginosa PAO1 in Whole Plant and Animal Pathogenicity Models. J. Agric. Food Chem. 2008, 56, 1955–1962.
- Rasmussen, T.B.; Bjarnsholt, T.; Skindersoe, M.E.; Hentzer, M.; Kristoffersen, P.; Köte, M.; Nielsen, J.; Eberl, L.; Givskov, M. Screening for Quorum-Sensing Inhibitors (QSI) by Use of a Novel Genetic System, the QSI Selector. J. Bacteriol. 2005, 187, 1799–1814.
- Bauer, W.D.; Mathesius, U. Plant responses to bacterial quorum sensing signals. Curr. Opin. Plant Biol. 2004, 7, 429–433.
- Gao, M.; Teplitski, M.; Robinson, J.B.; Bauer, W.D. Production of Substances by Medicago truncatula that Affect Bacterial Quorum Sensing. Mol. Plant-Microbe Interact. 2003, 16, 827–834.
- Kusari, P.; Kusari, S.; Spiteller, M.; Kayser, O. Implications of endophyte-plant crosstalk in light of quorum responses for plant biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 5383–5390.
- Teplitski, M.; Mathesius, U.; Rumbaugh, K.P. Perception and Degradation of N-Acyl Homoserine Lactone Quorum Sensing Signals by Mammalian and Plant Cells. Chem. Rev. 2011, 111, 100–116.
- Zhang, W.; Luo, Q.; Zhang, Y.; Fan, X.; Ye, T.; Mishra, S.; Bhatt, P.; Zhang, L.; Chen, S. Quorum Quenching in a Novel Acinetobacter sp. XN-10 Bacterial Strain against Pectobacterium carotovorum subsp. carotovorum. Microorganisms 2020, 8, 1100.
- Weller, D.M.; Mavrodi, D.V.; Van Pelt, J.A.; Pieterse, C.M.J.; Van Loon, L.C.; Bakker, P.A.H.M. Induced Systemic Resistance in Arabidopsis thaliana Against Pseudomonas syringae pv. tomato by 2,4-Diacetylphloroglucinol-Producing Pseudomonas fluorescens. Phytopathology 2012, 102, 403–412.
- Groenhagen, U.; Baumgartner, R.; Bailly, A.; Gardiner, A.; Eberl, L.; Schulz, S.; Weisskopf, L. Production of Bioactive Volatiles by Different Burkholderia ambifaria Strains. J. Chem. Ecol. 2013, 39, 892–906.
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012.
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932.
- Bais, H.P.; Park, S.-W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32.
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83.
- Zhang, Y.; Ruyter-Spira, C.; Bouwmeester, H.J. Engineering the plant rhizosphere. Curr. Opin. Biotechnol. 2015, 32, 136–142.
- Oldroyd, G.E.D. Speak, friend and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263.
- Downie, J.A. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol. Rev. 2010, 34, 150–170.
- Rios, J.; Pascual, J.; Guillen, M.; Lopez-Martinez, A.; Carvajal, M. Influence of foliar Methyl-jasmonate biostimulation on exudation of glucosinolates and their effect on root pathogens of broccoli plants under salinity condition. Sci. Hortic. 2021, 282, 110027.
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700.
- Pérez-Montaño, F.; Alias-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336.
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440.
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737.
