Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Because of their medicinal characteristics, effectiveness, and importance, plant-derived flavonoids have been a possible subject of research for many years, particularly in the last decade. Plants contain a huge number of flavonoids, and Diosmin, a flavone glycoside, is one of them. Numerous in-vitro and in-vivo studies have validated Diosmin’s extensive range of biological capabilities which present antioxidative, antihyperglycemic, anti-inflammatory, antimutagenic, and antiulcer properties.
- Diosmin
- target pathways
- anti-inflammatory
- anti-oxidant
- anti-cardiovascular
- anti-diabetic
1. Anti-Oxidant Property
Oxidative stress has been linked to the development of various diseases, including myocardial ischemia, cerebral ischemia–reperfusion injury, diabetes, neuronal cell injury, hypoxia, and cancer. In rat models, Diosmin was found to exhibit anti-oxidant effects and to stimulate human neutrophils. Because of its anti-oxidant properties, Diosmin has been shown to offer a variety of therapeutic effects for illnesses characterized by oxidative stress [1][2][3][4][5][6][7][8][9]. Diosmin was found to have significant anti-diabetic effects in diabetic rats fed streptozotocin nicotinamide (STZ-NA). When diabetic control rats were compared to normal control rats, anti-oxidant enzyme activities, such as glutathione-S-transferase (GST), glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT), as well as levels of low molecular weight antioxidants, such as vitamin C, vitamin E, and reduced glutathione (GSH), were found to be low, whereas lipid peroxidation Hyperglycemia with a significant drop in plasma insulin levels was also seen [10][11][12].
Oral administration of Diosmin enhanced the glycemic and anti-oxidant status of diabetic rats, according to previous studies. Diosmin treatment was also found to reduce lipid peroxidation [1][13][14]. Due to its anti-oxidant properties, Diosmin functions as an anti-hypertensive drug in deoxycorticosterone acetate (DOCA)-salt-induced hypertensive rats. Hypertension is characterized by an excessive production of reactive oxygen species. In DOCA-salt-treated rats, non-enzymatic and enzymatic antioxidants were found to be lower, while lipid peroxidation products (thiobarbituric acid reactive substances, lipid hydroperoxides, and conjugated dienes) were found to be significantly higher in blood plasma and tissues, such as the liver, kidney, heart, and aorta. Antioxidant levels were restored to near-normal levels, and lipid peroxidation products were reduced as a result of the Diosmin therapy. These findings were confirmed by histopathological examinations of the kidney and heart [11][15][16][17] Diosmin was found to have a hepatoprotective effect against ferrous sulfate-induced liver damage in adult male albino rats. Too much iron causes oxidative stress, reactive oxygen species (ROS), lipid peroxidation, inflammation, and tissue necrosis. Elevated ALT, AST, ALP, GGT, LDH activity, and bilirubin levels indicate hepatocyte membrane damage [13]. After Diosmin treatment, these values were considerably normalized. Because it maintains membrane integrity, decreases oxidative stress, and aids in the correction of dyslipidemia, Diosmin is a good hepatoprotective drug. The antioxidant and anti-inflammatory properties of Diosmin are primarily responsible for its hepatoprotective effects [11]. According to Senthamizhselvan et al., pre-treatment with Diosmin reduces oxidative stress in the rat heart after ischemia/reperfusion [18]. Ischemia–reperfusion injury occurs when ischemic tissue is reperfused, causing oxidative stress and cellular damage. The activities of enzyme antioxidants (SOD, CAT, and GPx) and GSH levels declined when hearts of control rats (no Diosmin pre-treatment) were subjected to an ischemia/reperfusion regimen, although the levels of lipid peroxidation products rose [19][20]. After 7 days of oral therapy with Diosmin (50 and 100 mg/kg), the activities of enzymatic antioxidants and GSH levels were found to be increased, while the levels of lipid peroxidation products were found to be reduced [18][21]. Ischemia–reperfusion injury produces edema and tissue destruction in the retina, resulting in vision loss. Diosmin was discovered to help male Wistar rats with retinal edema by maintaining the blood-retinal barrier and lowering vascular permeability. Diosmin’s ability to change the VEGF/PEDF ratio could explain its protective properties. The levels of malondialdehyde (MDA) and the activity of total-superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in retinal tissues that had been altered by ischemia–reperfusion injury, recovered to normal after Diosmin administration (Tong et al., 2013). Diosmin protects male CD-1 mice from brain ischemia–reperfusion injury by stimulating the JAK2/STAT3 signaling pathway [22][23]. In human polymorphonuclear neutrophils stimulated in vitro by phorbol myristate acetate, Cypriani et al. discovered that S5682 (Daflon 500 mg), a purified flavonoid fraction including Diosmin and hesperidin in a 9:1 ratio, has anti-oxidative effects (Cypriani et al., 1993). Diosmin’s free-radical scavenging activity is one of the reasons for its protection against myocardial infarction [17][24].
2. Anti-Inflammatory Property
Many diseases, including arthritis, allergies, asthma, autoimmune diseases, atherosclerosis, diabetes, and cancer, are caused by inflammation. Inflammation can be detected by measuring the levels of certain biomarkers known as inflammatory markers. Immune cells, such as neutrophils, basophils, eosinophils, platelets, macrophages, and others, cell surface receptors and adhesion molecules such as selectins (L-selectin, P-selectin, and E-selectin), and soluble mediators, such as cytokines (IL-1, IL-2, IL-6, TNF-α, TGF-ß, and IFN-γ), chemokines, and NF-kB (complement factors, C-reactive protein and the coagulation factor fibrinogen) [25][26][27][28][29].
Diosmin has been shown in multiple studies to lower these markers due to its anti-inflammatory effects. In lung damage produced by lipopolysaccharide (LPS) therapy, pro-inflammatory cytokines (IL-2, IL-6, IL-17, and TNF-α) and NF-kB were shown to be increased. After a few days of Diosmin pre-treatment, the levels of these indicators were dramatically lowered. In this study, male adult Balb/c mice were employed (Imam et al., 2015). In rat colitis caused by trinitrobenzenesulfonic acid, Diosmin was found to decrease the production of LTB4 (eicosanoid) and colonic MDA (TNBS). In the TNBS-treated colon, LTB4 improves neutrophil chemotaxis, adhesion, and degranulation, whereas MDA is a lipid peroxidation product (Crespo et al., 1999). According to Tahir et al., Diosmin reduces COX-2 and iNOS levels in chemically induced hepatocarcinogenesis (inflammatory markers). In female Wistar rats, diethylnitrosamine (DEN) was utilized to induce hepatocarcinogenesis, while 2-acetylaminofluorene was employed to promote it (2-AAF). In a long-term experiment, Diosmin was given orally at doses of 10 and 20 mg/kg b.wt for 9 weeks (Tahir et al., 2013). Diosmin treatment was found to alter the levels of tumor necrosis factor (TNF-α) and cyclooxygenase-2 in acetic acid-induced ulcerative colitis (COX-II). TNF-α and COX-II levels were lowered in a dose-dependent manner in Diosmin-treated rats [27].
3. Anti-Cancer Property
Recent study has discovered that Diosmin has dose-dependent pro-apoptotic effects on a range of animal cancers, including breast, prostate, colon, oral, and urinary bladder tumors. Diosmin causes premature senescence and apoptosis in MCF-7 cells at various doses. Other breast cancer cell lines, including MDA-MB-231 and SKBR-3, responded to Diosmin, but MCF-7 was found to be the most responsive. At lower doses, Diosmin produced G2/M cell cycle arrest, elevated p53, p21, and p27 levels, increased SA—gal activity, oxidative stress, and DNA damage in MCF-7 cells, all of which are associated with ageing. Apoptosis can be triggered by increased levels of nitric oxide, total ROS, total superoxide, mitochondrial production, and protein carbonylation. In research on the androgen-independent prostate cancer cell line DU145, Diosmin’s pro-apoptotic activity was confirmed. The genome and cytotoxicity of three flavonoid glycosides (Diosmin, naringin, and hesperidin) were studied in the DU145 prostate cancer cell line. The most genotoxicity was caused by Diosmin. In DU145 cells, these flavonoids caused oxidative stress or intracellular redox disequilibrium, leading to changes in mitochondrial membrane potential and apoptotic cell death. Diosmin dramatically boosted overall ROS production. Diosmin therapy also increased the amount of double stranded breaks in DNA and the formation of micronuclei (genotoxicity) [30]. Diosmin has anti-proliferative effect in human colon cancer cell lines, according to Kuntz et al. [31]. Tanaka et al. discovered a chemopreventive effect of Diosmin on colon carcinogenesis produced by azoxymethane in male F344 rats [32][33]. The treatment of Diosmin orally reduced colon carcinogenesis, as evidenced by lower rates of colon cancers. They hypothesized that inhibiting ornithine decarboxylase (ODC), a rate-limiting enzyme in polyamine production, was responsible for the decrease in colonic cancers [33]. Cell apoptosis is produced by DNA damage when ODC is inhibited [34]. When exposed to carcinogens, ODC levels have been reported to rise in numerous tissues. ODC activity was likewise elevated in the colonic mucosa of azoxymethane-treated rats. Diosmin has a dose-dependent cytotoxic capability for A431 skin cancer cells, according to Buddhan et al. It inhibits the invasive capacity of A431 cells by inducing apoptosis through a ROS-mediated mechanism [12][13]. DNA fragmentation, overexpression of p53, caspase 3 and caspase 9 genes, and downregulation of Bcl-2, matrix metalloproteinases-2 and 9 genes were all observed in A431 cells after Diosmin treatment. Diosmin’s IC50 value was discovered to be 45 g/mL, at which point it produced significant ROS [35]. Diosmin was found to prevent oral carcinogenesis produced by 4-nitroquinoline 1-oxide in male F344 rats (4-NQO). They theorized that Diosmin had anti-cancer capabilities via a number of mechanisms. One of these mechanisms was the inhibition of ornithine decarboxylase (ODC). Agents that inhibit ODC activity have been shown to be effective in slowing tumor growth. Another hypothesized mechanism is the inhibition of DT-diaphorase activity, which is required for 4-NQO to have its carcinogenic effect [32][33]. Inhibiting oral carcinogenesis, Diosmin is more efficient than diosmentin (the aglycone version of Diosmin) [36]. Oral treatment of 1000 ppm Diosmin suppressed urinary-bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl) nitrosamine in male ICR mice. These findings were supported by a count of silver-stained nucleolar-organizer-region-associated proteins (AgNORs) and a 5-bromodeoxyuridine (BUdR)-labeling index. Diosmin’s anti-carcinogenic effects may be due to a decrease in cell proliferation [23][37]. In male Wistar rats, Diosmin had a similar effect on esophageal carcinogenesis induced with N-methyl-N-amylnitrosamine (MNAN). Diosmin’s chemopreventive effects on several cancer kinds or cell lines are summarized in Figure 1.
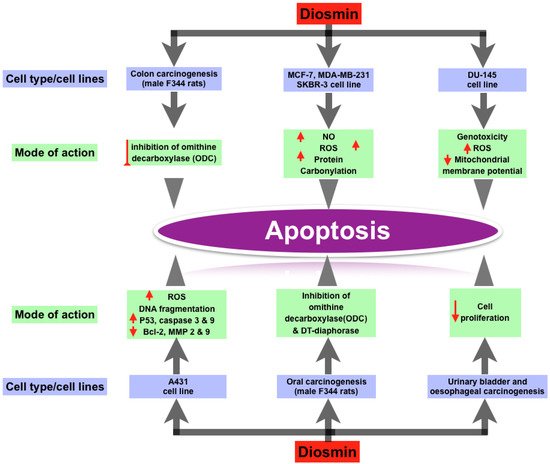
Figure 1. Effect of Diosmin on different types of cancer cell lines and their mode of actions.
Diosmin has also been shown to have anti-metastatic properties in metastatic lung melanoma (B16F10) [38]. Diosmin lowers the number of metastatic nodules, implant percentage, and invasion index in both micro- and macroscopic investigations. In the treatment of metastatic pulmonary melanoma, Diosmin acts in tandem with IFN-γ [39][40]. By disrupting the PI3K–Akt–MDM2 signaling pathway, Diosmin decreased HA22T cell growth (human hepatocellular cancer) in nude mice models and triggered G2/M cell cycle arrest through p53 activation [41][42].
4. Anti-Diabetic Property
In the treatment of diabetes and its complications, Diosmin has been proven to have therapeutic effects. Diabetes mellitus is a chronic disease characterized by abnormal glucose, protein, and lipid metabolism due to a lack of or decreased insulin activity. Diosmin possesses anti-hyperglycemic effects, according to numerous studies. According to Pari et al., Diosmin (in various doses) taken orally for 45 days can improve glycemic control. In the study, male albino Wistar strain rats were given streptozotocin-nicotinamide to develop diabetes (STZ-NA) [1][14]. The effect of Diosmin on plasma glucose levels was shown to be dose-dependent. In addition, oral Diosmin (100 mg/kg b.w.) treatment decreased glycosylated hemoglobin while raising hemoglobin and plasma insulin. Hexokinase and glucose-6-phosphate dehydrogenase, two essential liver enzymes, were also suppressed. In addition, there was a rise in body weight [1][14].
Diosmin has also been linked to improved lipid metabolism in people with diabetes. Hypercholesterolemia, lipid buildup in hepatic organs, and alterations in plasma lipid and lipoprotein profiles are all symptoms of metabolic dyslipidemia in people with type-2 diabetes [43][44]. Plasma lipids, tissue lipids (cholesterol, TGs, FFAs, and PLs), and plasma lipoproteins can all be efficiently normalized with Diosmin therapy (LDL, VLDL) [1][14]. Diosmin also corrects changes in glycoprotein profile caused by type-2 diabetes. When glucose is utilized by insulin-independent processes in diabetic people, glycoproteins build up. In STZ-NA-induced diabetic rats, the level of plasma glycoproteins increased dramatically. In diabetic rats’ liver and kidneys, hexose, hexosamine, and fucose levels were much greater, but sialic acid levels were significantly lower. Diosmin, when taken orally, has been shown to counteract these changes in glycoprotein profile [1][14].
Neuropathy is one of the most common diabetic consequences, affecting more than half of those who have the illness. Chronic untreated and uncontrolled hyperglycemia causes pain, tingling, and numbness in the periphery, as well as delayed nerve conduction. Hyperglycemia-induced oxidative stress results in the accumulation of polyols and advanced glycation end products, as well as impairment of (Na+/K+)-ATPase activity and endothelial function. Apoptosis is the death of neurons as a result of oxidative stress. Jain et al. employed streptozotocin and a high-fat diet to induce type-2 diabetes in male Sprague-Dawley rats to assess the effect of Diosmin on diabetic neuropathy. In rats, four weeks of treatment with Diosmin (50 and 100 mg/kg, p.o.) reduced the development of early diabetic neuropathy. It reduced oxidative stress by restoring GSH, NO, and SOD activity that had been changed [12][16][17][45]. Diosmin treatment resulted in considerable NF-kB normalization in alloxan-induced diabetic Wistar mice. NF-kB is important in the pathogenesis of diabetic neuropathy and other inflammatory illnesses [25]. In male Swiss mice, Diosmin was discovered to alleviate neuropathic pain produced by chronic constriction injury (CCI). Diosmin (1 or 10 mg/kg) was given intraperitoneally to reduce CCI-induced mechanical and thermal hyperalgesia. The role of the NO/cGMP/PKG/KATP channel signaling pathway was verified using inhibitors, such as L-NAME (an inhibitor of NOS), ODQ (an inhibitor of soluble guanylate cyclase), KT5823 (an inhibitor of PKG), or glibenclamide (an ATP-sensitive K+ channel blocker). Diosmin treatment also inhibited the production of cytokines (IL-1 and IL-33/St2) and reduced the activation of glial cells [16][46][47].
5. Anti-Bacterial Property
In bacteria, the majority of medications have developed resistance. Plants are being researched in the hope of discovering new and effective antibacterial agents. Plant-based bioactive components that are effective against fungus, yeasts, and bacteria, as well as insects, nematodes, and other plants, are known as phytochemicals. They can inhibit the synthesis of peptidoglycans, damage microbial membrane structures, alter the hydrophobicity of bacterial membrane surfaces, and interfere with quorum sensing (QS). By synthesizing silver nanoparticles of Diosmin, it has been shown the antibacterial properties (AgNPs) where they have tested its antimicrobial activity against Escherichia coli, Pseudomonas putida, and Staphylococcus aureus using the disc diffusion method. Diosmin produced hexagonal AgNPs that were slightly antimicrobial and had a size of roughly 5–40 nm. Diosmin inhibited E. coli, P. putida, and S. aureus with zones of inhibition of 6, 6, and 7 mm, respectively. Pits on the bacterial cell wall, changes in cell membrane permeability, obstruction of transduction, suppression of respiratory enzyme function due to free radical generation, and inactivation of various thiol-containing enzymes have all been proposed as antibacterial mechanisms [23][37][48].
Diosmin with amoxicillin-clavulanic acid (AMC) has been found to have mycobactericidal efficacy against Mycobacterium marinum. After treatment with a combination of AMC and Diosmin, the survival of M. marinum-infected Drosophila melanogaster fly models improved by 60%, providing in vitro proof. Its antibacterial activity was also proven against Mtb H37Ra and an MDR clinical isolate. The AMC-Diosmin combination was discovered to target L, D-transpeptidase (LDT) enzymes involved in Mtb cell wall synthesis, resulting in cellular leakage in M. marinum cells [49].
6. Cardiovascular Protection
Diets high in flavonoids help promote cardiovascular health. Diosmin has been shown to have anti-platelet action. Sulfation of Diosmin will very certainly improve overall binding at the heparin binding site, which contains helix A, D, and N-terminal residues, resulting in the development of novel anti-thrombin candidates. Diosmin’s anti-thrombotic properties are confirmed by changes in the protein composition of rats with venous thrombosis. Diosmin, which targets centrosome-associated protein 350, may increase endothelial cell proliferation (CEP350). In a rat model produced by the nitric oxide production inhibitor L-NAME, Diosmin’s antihypertensive effects are demonstrated [10][11][50][51][52][53][54][55]. Diosmin appears to protect rats from myocardial infarctions, hyaline arteriopathy, and fibrinoid necrosis caused by L-NAME. The elimination of superoxide anions by Diosmin could be the underlying mechanism for its anti-hypertensive actions. Diosmin has been shown to lower serum cardiac marker enzyme production, reduce plasma lipid peroxidation, and restrict lipid metabolism abnormalities in isoproterenol-induced myocardial-infarcted rats, resulting in anti-hyperlipidemia and cardio-protection. Isoproterenol has been reported to raise cardiac diagnostic markers, heart mitochondrial lipid peroxidation, and calcium ions while lowering anti-oxidant enzyme expression. Diosmin has been shown to be useful in preventing these traits. Left ventricular hypertrophy (LVH), ATPase dysfunction, and electrolyte imbalance all play a role in the etiology of isoproterenol-induced myocardial infarction. Pretreatment with Diosmin may attenuate the degenerative effects of isoproterenol in rats [10][11][16][46][50][56].
Reperfusion of ischemic tissues typically causes the generation of free radicals. Heart function recovery, anti-oxidant enzyme expression, and lipid peroxidation are all protected by Diosmin. In the presence of reperfusion stress, Diosmin successfully preserves TCA cycle enzyme activity. Diosmin reduces metabolic syndrome-related cardiovascular issues in rats, as demonstrated by improvements in systolic and diastolic blood pressure (BP) and ECG parameters. The anti-oxidant and anti-inflammatory effects of Diosmin in rats could explain these findings. Diosmetin protects mice from damage caused by ISO by upregulating AKT and NRF2 signaling while suppressing the NF-kB pathway. In venous smooth muscle action, Ca2+ is a key mediator. The contraction of the inferior vena cava (IVC) in normal Krebs and Ca2+-free Krebs has been shown to be unaffected by Diosmin. Diosmin, on the other hand, enhances the contractile response generated by KCl [41][57].
Sclerotherapy is a telangiectasia and varicose veins treatment that might cause irreversible endothelial damage. An exaggerated inflammatory response is usually induced during the sclerotherapy technique. MPFF has been shown to be useful in lowering inflammatory stress during sclerotherapy. By increasing venular diameter, preserving functional capillary density, reducing the number of leaky sites, and binding leukocytes, MPFF reduces post-sclerotherapy inflammation in a microvascular network. MPFF has been shown to lower the production of metalloproteinase-2 (MMP-2) and MMP-9 in rats while boosting MDA, which helps with varicose vein therapy. Linfadren, a combination of Diosmin, coumarin, and arbutin, has been shown in a randomized controlled trial to treat chronic hand edema in patients with post-trauma/surgery. Linfadren has been demonstrated to assist patients with breast cancer-related lymphedema when used in conjunction with extensive decongestive therapy [13][15][17][58].
Primary reflux from primary valve incompetence and venous thrombosis induces chronic venous illness, which includes pain, edema, skin damage, and ulceration. Two possible explanations are venous hypertension and microcirculation problems. Inflammation is both the beginning and the end of primary valve incompetence, as well as venous hypertension. Daflon 500 mg relieves clinical symptoms by reducing inflammatory reactions not only in the microcirculation but also in the vein wall and valve cusps. According to randomized trials, Daflon 500 mg for 60 days of therapy is also effective in elastic compression and speeding up the healing process in venous ulcers. A meta-analysis of Daflon 500 mg’s effects on venous leg ulcers found that it can be a valuable addition to standard therapy in big and long-standing ulcers. In randomized, double-blind, controlled trials, the therapeutic efficacy of Daflon 500 mg on chronic venous disease symptoms and edema was also studied. Diosmin may significantly reduce angiogenesis and inflammation, as evidenced by downregulation of TNF-α, IL-6, VEGF-C, VEGF-A, and FGF2 expression and overexpression of angiostatin expression in individuals with chronic venous issues. After six months of Daflon treatment, however, air plethysmography revealed no changes in venous hemodynamics. By magnifying adrenergic impact on microcirculation, Diosmin may cause adverse effects by raising creatine phosphokinase and serum lactic dehydrogenase levels [50][59][60][61].
Finally, Diosmin may protect against myocardial infarctions, hyaline arteriopathy, and fibrinoid necrosis caused by L-NAME by reducing serum cardiac marker enzyme production, plasma lipid peroxidation, and lipid metabolism alterations. By correcting isoproterenol-induced LVH, ATPase failure, and electrolyte imbalance, it improves metabolic syndrome-related cardiovascular issues. MPFF decreases MMP-2 and MMP-9 expression while increasing MDA, improving venular diameter, maintaining functional capillary density, reducing leaky sites, and sticking leukocytes. Inflammatory responses in the microcirculation, vein wall, and valve cusps are reduced with Daflon 500 mg. Daflon 500 mg has also been shown to be effective in the treatment of chronic venous disease symptoms and edema. TNF, IL-6, VEGF-C, VEGF-A, and FGF2 expression were all downregulated, whilst angiostatin expression was upregulated, showing that Diosmin reduces angiogenesis and inflammation in chronic venous disease patients. As evidenced by increases in SOD, CAT, GSH, and NO, as well as a decrease in MDA, Diosmin maintains redox equilibrium and downregulates the NF-B signaling pathway. Diosmin also lowers kidney weight, pH, total protein, calcium, and phosphorus in the urine, as well as potassium, sodium, magnesium, creatinine, and uric acid in the blood.
7. Liver Protection
Hypoxia, angiogenesis, inflammation, and intrapulmonary vasodilation characterize hepatopulmonary syndrome (HPS), a severe consequence of hepatic cirrhosis. TNF/VEGF, IGF-1/PI3K/AKT, and FGF-1/ANG-2 signaling pathways may be involved in HPS development and can be restored by Diosmin therapy in a chronic bile duct ligation (CBDL)-induced rat model. According to another study, Diosmin decreases BDL-induced liver abnormalities through modifying the Keap-1/NRF2 and p38-MAPK/NF-B/iNOS signaling pathways. Diosmin, pentoxifylline, and their combination have been shown to reduce BDL-induced liver cirrhosis via modulating the Keap-1/Nrf-2/GSH and NF-kB/p65/p38-MAPK signaling pathways. Diosmin also stimulates the production of cytoglobin, which contributes to the compound’s anti-oxidant, anti-inflammatory, and anti-fibrotic properties. Diosmin promotes the NRF2/Keap-1 pathway while suppressing the ROS-induced p38 MAPK pathways and activating the eNOS gene. By binding to the ARE sequence and upregulating the production of target genes, such as HO-1 and SOD, active NRF2 may enter the nucleus to promote transcriptional activity. The activity of the NF-kB, p53, and iNOS signaling pathways may be boosted when the p38 MAPK is triggered [10][12][14][21][23].
8. Neuroprotection
Diosmin performs a variety of actions that are linked to neuroprotection. Diosmin is a sedative that induces sleep and decreases the expression of IL-1, TNF, and IL-33/St2, as well as activating glial cells. It lowers mechanical and thermal hyperalgesia by activating D2, GABAA, and opioid receptors but not 5-HT1A receptors, reduces neuropathic pain by activating the NO/cGMP/PKG/KATP pathway, and suppresses IL-1, TNF-a, and other inflammatory mediators. Diosmin has been associated to thermal hyperalgesia, cold allodynia, and walking dysfunctions, as well as oxidative stress. It also helps with scopolamine-induced neural plasticity disruption and cognitive deficits by inhibiting TNF expression. Diosmin has an IC50 of 12.24 0.54 g mL and interacts with AChE enzyme via Tyr72, Tyr124, Trp286, Phe295, and Tyr341. Many flavone derivatives have been discovered as possible GABAA receptor ligands in the central nervous system, where they interact with the benzodiazepine binding site to produce depressive effects. The activity of the GABAA receptor cannot be controlled directly by flavonoid glycosides. Diosmin has sedative and sleep-inducing properties that are unrelated to GABAA receptor modulation. 6-C-glycoside-diosmetin activation of the GABAA receptor has been shown to have memory-enhancing and anxiolytic-like effects, which are controversial. As a result, more research into flavone derivatives’ interactions with the GABAA receptor is required. A range of harmful compounds in the peripheral and central nerve systems induces neuropathic pain. Diosmin coupled with hesperidin effectively reduces mechanical or thermal hyperalgesia in the chronic constriction injury (CCI) rat model. D2, GABAA, and opioid receptor antagonists may prevent these effects, while the 5-HT1A receptor inhibitor does not. In a CCI rat model, Diosmin alone has been shown to have anti-hyperalgesic effects via D2 and opioid receptors, as well as a reduction in pro-inflammatory cytokine (IL-1, TNF, and IL-6) expression [10][46].
Misfolded proteins have been linked to neurodegenerative disorders. Diosmin was discovered to bind to hen egg white lysozyme (HEWL) in a sheet-shape, in an in vitro research, minimizing HEWL aggregation. These may play a role in treating amyloid-related illnesses. The synthesis and aggregation of amyloid-(A) peptide aids the degenerative course of Alzheimer’s disease (AD). Inhibiting GSK-3 using Diosmin and its aglycone derivative diosmetin has been demonstrated to reduce brain soluble A and A oligomer formation, as well as tau hyperphosphorylation, and improve cognitive impairment in rats. Diosmin may aid to ameliorate scopolamine-induced synaptic plasticity disruption and cognitive impairments by reducing the expression of the pro-inflammatory cytokine TNF in the rat hippocampus. Traumatic brain injury (TBI) can cause mental and cognitive disability. Diosmin inhibits the pro-inflammatory cytokine TNF-α, protecting against TBI-induced declines in neurological scores, memory, and long-term potentiation (Yizhou Zheng, 2020a).
Diosmin appears to protect PC12 cells from LPS-induced TNF production and apoptosis, as seen by decreased DNA fragmentation, decreased Bad and caspase-3 expression, and enhanced Bcl-2 expression. Diosmin interacts with critical residues Tyr72, Tyr124, Trp286, Phe295, and Tyr341 in the acetylcholinesterase (AChE) enzyme and has an IC50 value of 12.24 0.54 g mL−1. This has a similar binding orientation to Donepezil. Diosmin’s inhibitory impact on the AChE enzyme has been confirmed in silico and in vitro experiments. In an ELISA experiment using the Ellman method, Diosmin had no effect on the activity of AChE and butyrylcholinesterase (BChE).
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10051076
References
- Srinivasan, S.; Pari, L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem.-Biol. Interact. 2012, 195, 43–51.
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134.
- Pfeffer, P.E.; Lu, H.; Mann, E.H.; Chen, Y.-H.; Ho, T.-R.; Cousins, D.J.; Corrigan, C.; Kelly, F.J.; Mudway, I.S.; Hawrylowicz, C.M. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS ONE 2018, 13, e0200040.
- Zraika, S.; Hull, R.L.; Udayasankar, J.; Aston-Mourney, K.; Subramanian, S.L.; Kisilevsky, R.; Szarek, W.A.; Kahn, S.E. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia 2009, 52, 626–635.
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The effects of oxidative stress on the development of atherosclerosis. Biol. Chem. 2019, 400, 711–732.
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152.
- Hallak, M.; Vazana, L.; Shpilberg, O.; Levy, I.; Mazar, J.; Nathan, I. A molecular mechanism for mimosine-induced apoptosis involving oxidative stress and mitochondrial activation. Apoptosis 2008, 13, 147–155.
- De Las Heras, N.; Martín Giménez, V.M.; Ferder, L.; Manucha, W.; Lahera, V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants 2020, 9, 897.
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835.
- Zhang, Z.; Liu, Q.; Yang, J.; Yao, H.; Fan, R.; Cao, C.; Liu, C.; Zhang, S.; Lei, X.; Xu, S. The proteomic profiling of multiple tissue damage in chickens for a selenium deficiency biomarker discovery. Food Funct. 2020, 11, 1312–1321.
- Abdel-Reheim, M.A.; Messiha, B.A.S.; Abo-Saif, A.A. Hepatoprotective Effect of Diosmin on Iron-induced Liver Damage. Int. J. Pharmacol. 2017, 13, 529–540.
- Eraslan, G.; Sarıca, Z.S.; Bayram, L.Ç.; Tekeli, M.Y.; Kanbur, M.; Karabacak, M. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ. Sci. Pollut. Res. Int. 2017, 24, 27931–27941.
- Perumal, S. Effect of diosmin on apoptotic signaling molecules in N- nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Mol. Cell. Biochem. 2018, 449, 27–37.
- Pari, L.; Srinivasan, S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2010, 64, 477–481.
- Bogucka-Kocka, A.; Woźniak, M.; Feldo, M.; Kockic, J.; Szewczyk, K. Diosmin—Isolation techniques, determination in plant material and pharmaceutical formulations, and clinical use. Nat. Prod. Commun. 2013, 8, 545–550.
- Bozdağ, M.; Eraslan, G. The effect of diosmin against lead exposure in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 639–649.
- Adouani, I.; Qureshi, A.S.; Hang, T.-J. Preparation, evaluation and pharmacokinetics of diosmin herbosomein beagle dogs. Pak. J. Pharm. Sci. 2019, 33, 033–040.
- Senthamizhselvan, O.; Manivannan, J.; Silambarasan, T.; Raja, B. Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur. J. Pharmacol. 2014, 736, 131–137.
- Tong, N.; Zhang, Z.; Zhang, W.; Qiu, Y.; Gong, Y.; Yin, L.; Qiu, Q.; Wu, X. Diosmin Alleviates Retinal Edema by Protecting the Blood-Retinal Barrier and Reducing Retinal Vascular Permeability during Ischemia/Reperfusion Injury. PLoS ONE 2013, 8, e61794.
- Calvert, J.W. Chapter 5—Ischemic Heart Disease and its Consequences. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Willis, M.S., Homeister, J.W., Stone, J.R., Eds.; Cellular and Molecular Pathobiology of Cardiovascular Disease; Academic Press: San Diego, CA, USA, 2014; pp. 79–100.
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.A.; Iqbal, M.; Anwer, M.K.; Hoshani, A.A.R.; Attia, S.M.; Ahmad, S.F. Pharmacological Research. Pharmacol. Res. 2015, 102, 1–11.
- Liu, W.Y.; Liou, S.-S.; Hong, T.-Y.; Liu, I.-M. The Benefits of the Citrus Flavonoid Diosmin on Human Retinal Pigment Epithelial Cells under High-Glucose Conditions. Molecules 2017, 22, 2251.
- Kilit, A.C.; Kose, E.O.; Imir, N.G.; Aydemir, E. Anticancer and antimicrobial activities of diosmin. Genet. Mol. Res. 2021, 20, GMR18752.
- Queenthy, S.S.; John, B. Diosmin exhibits anti-hyperlipidemic effects in isoproterenol induced myocardial infarcted rats. Eur. J. Pharmacol. 2013, 718, 213–218.
- Ahmed, S.; Mundhe, N.; Borgohain, M.; Chowdhury, L.; Kwatra, M.; Bolshette, N.; Ahmed, A.; Lahkar, M. Diosmin Modulates the NF-kB Signal Transduction Pathways and Downregulation of Various Oxidative Stress Markers in Alloxan-Induced Diabetic Nephropathy. Inflammation 2016, 39, 1783–1797.
- Crespo, M.E.; Gálvez, J.; Cruz, T.; Ocete, M.A.; Zarzuelo, A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999, 65, 651–653.
- Shalkami, A.S.; Hassan, M.; Bakr, A.G. Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 2017, 37, 78–86.
- Ali, N.; AlAsmari, A.F.; Imam, F.; Ahmed, M.Z.; Alqahtani, F.; Alharbi, M.; AlSwayyed, M.; AlAsmari, F.; Alasmari, M.; Alshammari, A.; et al. Protective effect of diosmin against doxorubicin-induced nephrotoxicity. Saudi J. Biol. Sci. 2021, 28, 4375–4383.
- Yao, X.; Gu, X.; Jin, S.; Shi, K.; Gao, X.; Wang, Q.; Zhao, J.; Zhang, H.; Lai, X. Anticancer and Anti-inflammatory Effect of Diosmin against Dalton Ascitic Lymphoma Induced Leukemia. J. Oleo Sci. 2021, 70, 665–673.
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130.
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142.
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Fukutani, K.; Ogawa, H. Modulation of N-methyl-N-amylnitrosamine-induced rat oesophageal tumourigenesis by dietary feeding of diosmin and hesperidin, both alone and in combination. Carcinogenesis 1997, 18, 761–769.
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Tanaka, T.; Ogawa, H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 1997, 18, 957–965.
- Pendeville, H.; Carpino, N.; Marine, J.C.; Takahashi, Y.; Muller, M.; Martial, J.A.; Cleveland, J.L. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 2001, 21, 6549–6558.
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24.
- Browning, A.M.; Walle, U.K.; Walle, T. Flavonoid glycosides inhibit oral cancer cell proliferation—role of cellular uptake and hydrolysis to the aglycones. J. Pharm. Pharmacol. 2005, 57, 1037–1041.
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of silver nanoparticles using flavonoids: Hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016, 6, 173–181.
- Martínez, C.; Vicente, V.; Yáñez, J.; Alcaraz, M.; Castells, M.T.; Canteras, M.; Benavente-García, O.; Castillo, J. The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histol. Histopathol. 2005, 20, 1121–1129.
- Álvarez, N.; Vicente, V.; Martínez, C. Synergistic Effect of Diosmin and Interferon-α on Metastatic Pulmonary Melanoma. Cancer Biother. Radiopharm. 2009, 24, 347–352.
- Martínez Conesa, C.; Vicente Ortega, V.; Yáñez Gascón, M.J.; Alcaraz Baños, M.; Canteras Jordana, M.; Benavente-García, O.; Castillo, J. Treatment of Metastatic Melanoma B16F10 by the Flavonoids Tangeretin, Rutin, and Diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797.
- Dung, T.; Lin, C.-H.; Việt Bình, T.; Hsu, H.-H.; Su, C.-C.; Lin, Y.-M.; Tsai, C.-H.; Tsai, F.-J.; Kuo, W.-W.; Chen, L.-M.; et al. Diosmin induces cell apoptosis through protein phosphatase 2A activation in HA22T human hepatocellular carcinoma cells and blocks tumour growth in xenografted nude mice. Food Chem. 2012, 132, 2065–2073.
- Dung, T.D.; Day, C.H.; Binh, T.V.; Lin, C.-H.; Hsu, H.-H.; Su, C.-C.; Lin, Y.-M.; Tsai, F.-J.; Kuo, W.-W.; Chen, L.-M.; et al. PP2A mediates diosmin p53 activation to block HA22T cell proliferation and tumor growth in xenografted nude mice through PI3K–Akt–MDM2 signaling suppression. Food Chem. Toxicol. 2012, 50, 1802–1810.
- Farmer, J.A. Diabetic dyslipidemia and atherosclerosis: Evidence from clinical trials. Curr. Diabetes Rep. 2008, 8, 71–77.
- Shepherd, J. Does statin monotherapy address the multiple lipid abnormalities in type 2 diabetes? Atheroscler. Suppl. 2005, 6, 15–19.
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41.
- Fattori, V.; Rasquel-Oliveira, F.S.; Artero, N.A.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Verri, W.A. Diosmin Treats Lipopolysaccharide-Induced Inflammatory Pain and Peritonitis by Blocking NF-κB Activation in Mice. J. Nat. Prod. 2020, 83, 1018–1026.
- Zielinska, D.F.; Gnad, F.; Schropp, K.; Wiśniewski, J.R.; Mann, M. Short Article. MOLCEL 2012, 46, 542–548.
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013, 19, 792–800.
- Pushkaran, A.C.; Vinod, V.; Vanuopadath, M.; Nair, S.S.; Nair, S.V.; Vasudevan, A.K.; Biswas, R.; Mohan, C.G. Combination of Repurposed Drug Diosmin with Amoxicillin-Clavulanic acid Causes Synergistic Inhibition of Mycobacterial Growth. Sci. Rep. 2019, 9, 6800.
- Feldo, M.; Wójciak-Kosior, M.; Sowa, I.; Kocki, J.; Bogucki, J.; Zubilewicz, T.; Kęsik, J.; Bogucka-Kocka, A. Effect of Diosmin Administration in Patients with Chronic Venous Disorders on Selected Factors Affecting Angiogenesis. Molecules 2019, 24, 3316.
- Luepker, R.V. Cardiovascular disease: Rise, fall, and future prospects. Annu. Rev. Public Health 2011, 32, 1–3.
- Nabel, E.G. Cardiovascular disease. N. Engl. J. Med. 2003, 349, 60–72.
- Iafisco, M.; Alogna, A.; Miragoli, M.; Catalucci, D. Cardiovascular nanomedicine: The route ahead. Nanomedicine 2019, 14, 2391–2394.
- Martín Giménez, V.M.; Kassuha, D.E.; Manucha, W. Nanomedicine applied to cardiovascular diseases: Latest developments. Ther. Adv. Cardiovasc. Dis. 2017, 11, 133–142.
- Mashour, N.H.; Lin, G.I.; Frishman, W.H. Herbal medicine for the treatment of cardiovascular disease: Clinical considerations. Arch. Intern. Med. 1998, 158, 2225–2234.
- Stansfield, W.E.; Ranek, M.; Pendse, A.; Schisler, J.C.; Wang, S.; Pulinilkunnil, T.; Willis, M.S. Chapter 4—The Pathophysiology of Cardiac Hypertrophy and Heart Failure. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Willis, M.S., Homeister, J.W., Stone, J.R., Eds.; Cellular and Molecular Pathobiology of Cardiovascular Disease; Academic Press: San Diego, CA, USA, 2014; pp. 51–78.
- Soares, J.M.; Faria, B.M.D.; Ascari, L.M.; Souza, J.M.D.; Soares, A.G.; Cordeiro, Y.; Romão, L.F. Diosmin induces caspase-dependent apoptosis in human glioblastoma cells. An. Acad. Bras. Ciências 2019, 91, e20191031.
- Katsenis, K. Micronized purified flavonoid fraction (MPFF): A review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr. Vasc. Pharmacol. 2005, 3, 1–9.
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274.
- Munjal, A.; Khandia, R. Atherosclerosis: Orchestrating Cells and Biomolecules Involved in Its Activation and Inhibition; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 1–38.
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and cancer risk:new mechanistic insightsfrom epidemiology. Nat. Rev. Cancer 2015, 15, 484–498.
This entry is offline, you can click here to edit this entry!
