Leishmania are protozoa belonging to the Phylum Euglenozoa Cavalier-Smith, 1981; Class Kinetoplastea Honigberg, 1963; order Trypanosomatida Kent, 1880; family Trypanosomatidae Doeflein, 1901 and subfamily Leishmaniinae, Maslov and Lukes 2012. The genus Leishmania is further divided into four subgenera, L. (Leishmania) Safjanova 1982, L. (Viannia) Lainson and Shaw, 1987, L. (Mundinia) Shaw, Camargo and Teixeira 2016 and L. (Sauroleishmania) Ranque 1973 [1]. Members of the first three subgenera are causative agents of leishmaniasis, the worldwide distributed, vector-borne human and veterinary disease. The main insect vectors are phlebotomine sand flies (Diptera: Phlebotominae).
Human leishmaniases are highly variable in their clinical manifestation, ranging from self-healing cutaneous lesions to serious visceral forms, life-treating if untreated. The main representative of the zoonotic cutaneous leishmaniasis in the Old World is Leishmania major, distributed from North and West Africa through Sahel belt and the Middle East to Central Asia, Mongolia and south-west Asia. This species is transmitted by sand flies of the subgenus Phlebotomus (Phlebotomus). The sores appear at the site of insect bite and the necrotic process results in large, open and wet lesions which cure without treatment. Several lesions may occur simultaneously. Reservoir hosts of L. major are various rodents, humans getting infected incidentally. Leishmania donovani is the causative agent of visceral leishmaniasis, called also kala azar. Fully developed kala azar is characterized with anaemia, haepatosplenomegaly and progressive cachexia and may be fatal if untreated, but subclinical or asymptomatic cases are frequent. The main vectors are P. (Larrousius) orientalis in East Africa and Phlebotomus (Euphlebotomus) argentipes in the Indian peninsula. The disease is regarded as anthroponotic in Indian peninsula while involvement of reservoir animals has been suggested in East Africa [2].
Espinosa, O. A., Serrano, M. G., Camargo, E. P., Teixeira, M. G., Shaw, J. J. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology 2016 doi:10.1017/S0031182016002092
Ashford, R.W. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 2000, 30:1269-1281.
- Leishmania donovani
- Leishmania major
- visceral leishmaniasis
- cutaneous leishmaniasis
- phlebotomine sand flies
- reservoir host
- model animals
- Phodopus sungorus
- Cricetulus griseus
- Lagurus lagurus
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Leishmania parasites (Kinetoplastida: Trypanosomatidae) are causative agents of leishmaniases, a group of diseases prevalent worldwide in 98 countries with more than 350 million people considered at risk, more than 1 million new cases occurring every year, and more than 50 thousand deaths annually, due to the visceral form [1,2]. The epidemiology and ecology of leishmaniases are exceptionally complex—at least 20 Leishmania species are pathogenic to humans, each of them possessing different mammalian reservoir hosts, as well as insect vectors [3].
This species diversity is reflected in the broad spectrum of clinical manifestations of human leishmaniases, which results from interactions between the parasite species and the host immune responses. Cutaneous leishmaniasis (CL), transmitted by diverse sand fly vector species, is caused principally by L. major, L. tropica, and L. aethiopica in the Old World and L. mexicana, L. venezuelensis, L. amazonensis, L. braziliensis, L. panamensis, L. guyanensis, and L. peruviana in the New World. Infection with CL may be characterized by localized, diffuse, or disseminated skin lesions [4]. Metastatic mucocutaneous leishmaniasis (MCL), confined to the New World, is due to Leishmania (Viannia) braziliensis or, less frequently, L. (V.) panamensis and L. (V.) guyanensis. Visceral leishmaniasis (VL), caused by L. donovani and L. infantum, is the most severe form, often fatal if left untreated, characterized by fever, loss of weight, splenomegaly, hepatomegaly and/or lymphadenopathy, and anemia [5].
Experimental animal models are expected to mimic the specific features of the variety of human leishmaniases. Many immunological aspects of the disease have been studied using standard laboratory models, such as mice, hamsters, domestic dogs, and non-human primates. However, none of them accurately reproduces the outcome of human Leishmania infection [6]. The major advantage of inbred mouse models is their controlled genetic background and well-defined immune response. On the other hand, replication and spread of the pathogen in mice are far from the natural pattern. The relative lack of genetic polymorphism in laboratory mice has been specifically overcome by using stocks derived from recently trapped wild progenitors belonging to different taxa of the genus Mus [7]. An alternative approach is the use of genetically polymorphic wild rodents as experimental animal models for host-parasite relationships studies. These models allow a better understanding of the dynamics and range of infection, including mechanisms of parasite amplification, their availability for transmission, and the natural regulation of the immune response [6].
More than 20 rodent species have been used for experimental infections with Leishmania parasites. The extensive research in this field was mainly done during the first half of the last century. However, many of the tested species are protected or difficult to breed in the laboratory, and many of them have been shown to be resistant to infection (reviewed in [8]). More recently, two New World wild rodent species Thrichomys laurentius and Peromyscus yucatanicus have been used for the study of L. infantum, L. braziliensis, and L. mexicana infections, respectively [9,10].
2. Development of L. major in Four Rodent Species
In total, 12 BALB/c mice, 12 P. sungorus, 16 C. griseus, and 16 L. lagurus were infected; half of them with 1 × 105 culture-derived parasites (CDP) selected with PNA for high representation of metacyclic forms and the second half with 6–7 × 104 sand fly-derived parasites (SDP), where metacyclics comprised 69–73% of all morphological forms. The numbers of SDP were derived from 10 dissected sand fly females, without any adjustments, to keep the character of the inoculum as natural as possible.
All four rodent species showed stable growth of the body weight during the experiments, and no significant differences in weight gains were found between the groups infected with CDP and SDP (Table S1). In addition, the lesion growth was very similar for the two inoculum types in BALB/c mice, P. sungorus, and L. lagurus. In C. griseus, lesions in the SDP inoculated group developed more slowly, but the difference against the CDP infected group did not reach the statistical significance (Figure 1 and Table S2).
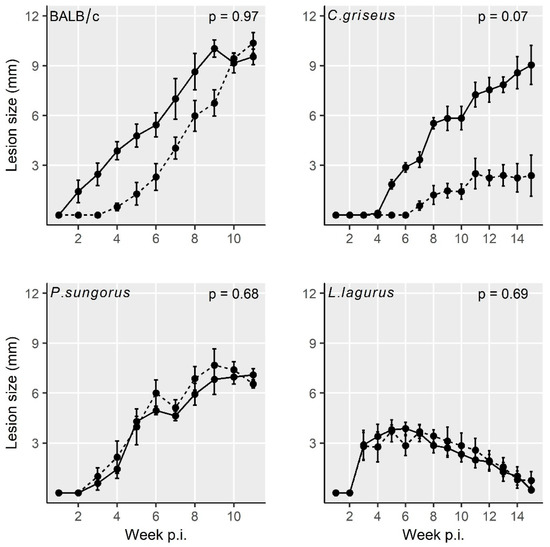
Figure 1. Lesion growth in animals inoculated with L. major using the two infection modes. Solid line = infection with culture-derived promastigotes (CDP); dashed line = infection with sand fly-derived promastigotes (SDP). p values indicate the statistical difference between the two infection types. Data are presented as the means ± standard errors of the means; 15% of the variance was explained by individual variability.
Lesions appeared very early in BALB/c mice (already on week 2 p.i.), one week later in P. sungorus and L. lagurus (in week 3 p.i.), and even later in C. griseus (week 4 p.i.). Compared to susceptible BALB/c mice, the growth of lesions was very similar in P. sungorus (p = 0.08); the lesions have an ulcerative character in both species (Figure 2a,b), and their size increased progressively until the end of the experiment. In some P. sungorus, the skin surrounding ears was also affected by redness and exuviation (Figure 2c). Experiments with BALB/c mice and P. sungorus were, therefore, finished on week 9–11 p.i. to avoid excessive distress to the animals. In L. lagurus, the lesion growth was significantly slower than in BALB/c mice (p < 0.0001), and although the character of lesions was initially also ulcerative (Figure 2d); their size increased only to week 7 p.i. and then began to reduce. These animals were able to resolve lesions by necrosis of affected parts, resulting in a reduction of the size of ear pinnae (Figure 2e). In C. griseus, lesion development was also significantly slower than in BALB/c mice (p = 0.0001); lesions were not ulcerative and were fully healed in some (3 out of 13) animals (Figure 2f, Table S2).
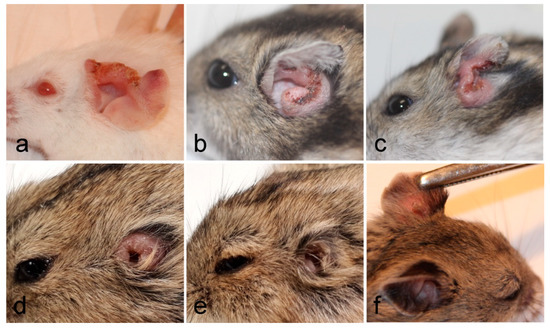
Figure 2. Inoculated ears of BALB/c mice (a), P. sungorus (b,c), L. lagurus (d,e), and C. griseus (f) showing external manifestation of L. major infections at the end of experiments.
Numbers of parasites in inoculated ears were evaluated from the samples taken post-mortem using two different methods—qPCR and flow cytometry. Similar to the previous analyses, qPCR did not reveal significant differences between animals inoculated with CDP and SDP; therefore, results were summarized for both infection modes. Parasite loads in inoculated ears generally ranged between 102 and 107 parasites with a median of 103–104 in L. lagurus and 105–106 in the remaining three species. Both quantification methods showed similar numbers of parasites in inoculated ears of BALB/c mice and P. sungorus (Table 1 and Figure S1). Based on the data from qPCR, significantly lower parasite loads in comparison with numbers in BALB/c mice were detected in C. griseus (p = 0.03), and the parasite load was lowest in L. lagurus (p = 0.0003). Based on data from flow cytometry, the difference between the four species was insignificant in this respect. The direct comparison of the two quantification methods in individual species revealed no significant differences in BALB/c mice (p = 0.6221), P. sungorus (p = 0.4357), and C. griseus (p = 0.08), while in L. lagurus qPCR gave significantly lower numbers than flow cytometry (p = 0.011).
Table 1. Comparison of L. major numbers detected in inoculated ears by flow cytometry (FC) and qPCR (PCR). The numbers represent parasite loads in half of the ear pinna.
| Rodent Species | No. of Samples | Median (in Thousands) |
Minimum (in Thousands) |
Maximum (in Thousands) |
p Values 1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FC | PCR | FC | PCR | FC | PCR | FC | PCR | FC | PCR | |
| BALB/c mice | 12 | 12 | 430 | 438 | 2 | 0.3 | 2756 | 14,270 | - | - |
| P. sungorus | 5 | 11 | 732 | 293 | 28 | 46 | 820 | 2240 | 0.93 | 0.19 |
| C. griseus | 13 | 16 | 744 | 152 | 6 | 0 | 1947 | 2240 | 0.89 | 0.03 |
| L. lagurus | 16 | 16 | 7 | 3 | 0.04 | 0.1 | 6982 | 974 | 0.09 | 0.0003 |
The distribution of parasites in rodent bodies was evaluated using the PCR and the fluorescence detection with In Vivo Xtreme (Table S3, Figure 3a, and Appendix A). In BALB/c mice, parasites remained restricted to both ears (inoculated and contralateral) and their draining lymph nodes. Only in one animal, the liver was also infected. In all remaining species, Leishmania parasites were detected in all the tested organs and tissues. In P. sungorus, the infection rates in all these tissues were the highest, and these hamsters were also the sole species where blood was also infected. Generally, the PCR was more sensitive than fluorescence detection. For example, numbers of parasites detected in contralateral ears by qPCR reached up to 86 thousand, 56 thousand, 31 thousand, and 15 thousand in BALB/c mice, P. sungorus, C. griseus, and L. lagurus, respectively, but the ears did not produce higher fluorescence signal than negative controls. In addition, the fluorescence detection was not applicable to densely haired paws of P. sungorus and L. lagurus, where even the control animals produced too strong a fluorescence signal (Appendix A). On the other hand, this method gave a good spatial picture of the parasite distribution. In ear pinnae, the fluorescence signal mostly corresponded to areas affected by skin lesions (Figure 3b). In the liver, the signal came either from the single site (typically in BALB/c mice or L. lagurus), or there were several smaller fluorescence centers dispersed over the whole organ (apparent in P. sungorus infected with CDP, Appendix A).
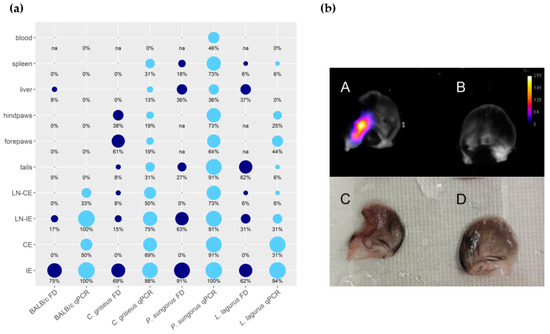
Figure 3. Anatomical distribution of L. major in four rodent species. (a) Results of fluorescence detection (FD, dark blue) and qPCR (light blue) presented in the balloon graph where the size of the balloon corresponds to the infection rate, i.e., the percentage of L. major-infected organs from the total sum of tested organs of the same type. IE = inoculated ears; CE = contralateral ears; LN-IE = draining lymph nodes of the inoculated ears; LN-CE = draining lymph nodes of the contralateral ears; na = not analyzed. (b) Ears of the C. griseus wetted with saline, photographed by the end of the experiment at week 15 p.i. A, B, images from In Vivo Xtreme optical display; C, D, images taken by Canon EOS 60D camera with Canon MP-E 65 mm f/2,8 1–5× Macro objective. A, C—inoculated left ear with ulcerative lesion and 2240 thousand parasites detected by q PCR. B, D—contralateral right ear with no external manifestation of the disease and 16.8 thousand parasites according to qPCR.
Infectiousness of animals, tested by xenodiagnosis experiments with P. duboscqi females, was, again, very similar in the groups infected with CDP and SDP (Table 2). Infectiousness corresponded very well to parasite loads in inoculated ears: The values in P. sungorus and C. griseus (29% and 11% of sand flies infected, respectively) did not differ significantly from infectiousness of BALB/c mice (14% of sand flies infected, p = 0.19 and p = 0. 54, respectively), while L. lagurus infected significantly fewer sand fly females than BALB/c mice (8% of sand flies infected, p = 0.006).
Table 2. Results of xenodiagnosis experiments performed with P. duboscqi and the four rodent species infected with L. major.
| Rodent Species | Rodent Numbe 1 | No. of Sand Fly Females | No. and (%) of Positive Females | Rodent Species | Rodent Number | No. of Sand Fly Females | No. and (%) of Positive Females |
|---|---|---|---|---|---|---|---|
| BALB/c mice p = 0.58 |
C1 | 30 | 0 | P. sungorus p = 0.80 |
C1 | 33 | 5 (15) |
| C2 | 32 | 7 (21) | C2 | 25 | 5 (20) | ||
| C3 | 24 | 3 (12) | C3 | 22 | 6 (27) | ||
| C4 | 30 | 4 (13) | C4 | 24 | 11 (46) | ||
| C5 | 29 | 8 (27) | C5 | 22 | 8 (36) | ||
| C6 | 30 | 5 (17) | ∑ | 126 | 35 (28) | ||
| ∑ | 175 | 27 (15) | S1 | 32 | 6 (19) | ||
| S1 | 32 | 6 (18) | S2 | 25 | 9 (36) | ||
| S2 | 30 | 5 (17) | S3 | 33 | 9 (27) | ||
| S3 | 31 | 8 (26) | S4 | 22 | 10 (45) | ||
| S4 | 34 | 0 | S5 | 23 | 6 (26) | ||
| S5 | 39 | 5 (13) | ∑ | 135 | 40 (30) | ||
| S6 | 33 | 0 | Total | 261 | 75 (29) | ||
| ∑ | 199 | 24 (12) | L. lagurus p = 0.36 |
C1 | 4 | 0 | |
| Total | 374 | 51 (14) | C2 | 2 | 0 | ||
| C. griseus p = 0.84 |
C1 | 23 | 6 (26) | C3 | 2 | 0 | |
| C2 | 24 | 3 (13) | C4 | 20 | 2 (10) | ||
| C3 | 27 | 2 (7) | C5 | 25 | 0 | ||
| C4 | 23 | 1 (4) | C6 | 23 | 1 (4) | ||
| C5 | 23 | 0 | C7 | 24 | 2 (8) | ||
| C6 | 24 | 5 (21) | ∑ | 100 | 5 (5) | ||
| C7 | 20 | 0 | S1 | 3 | 0 | ||
| ∑ | 164 | 17 (10) | S2 | 2 | 2 (100) | ||
| S1 | 28 | 0 | S3 | 2 | 0 | ||
| S2 | 15 | 5 (33) | S4 | 22 | 2 (9) | ||
| S3 | 26 | 0 | S5 | 18 | 1 (6) | ||
| S4 | 23 | 8 (35) | S6 | 13 | 3 (23) | ||
| S5 | 15 | 2 (13) | S7 | 26 | 3 (12) | ||
| S6 | 19 | 0 | S8 | 22 | 0 | ||
| ∑ | 126 | 15 (12) | ∑ | 108 | 11 (10) | ||
| Total | 290 | 32 (11) | Total | 208 | 16 (8) |
3. Development of L. donovani in Four Rodent Species
In total, 14 C. griseus, 12 BALB/c mice, 12 L. lagurus, and 12 M. auratus were infected with L. donovani; 3 L. lagurus and 3 M. auratus with 105 CDP, three individuals of each species with 107 CDP, and 32 animals with 5–63.9 × 104 SDP, where metacyclics comprised 29–46% of all morphological forms.
All the rodent species showed stable weight gain during the experiment, and the weight did not differ between the CDP and SDP groups (Table S4). None of the inoculated animals developed lesions or other external signs of the disease throughout the entire experiment. Nevertheless, qPCR performed at the end of the experiment (on week 30 p.i.) revealed the presence of L. donovani DNA in various tissues and organs of infected rodents (Table S5). The size of the inoculum (105 vs. 107 parasites) considerably influenced the outcome of the infection. In both species inoculated with 105 CDP (M. auratus and L. lagurus), only one of 3 individuals became infected, dissemination through the body was limited, and only low parasite loads (<103) were found in tissues of these animals (Figure 4a). Higher infection rates and parasite loads were detected in animals infected with 107 CDP and SDP, and no significant differences were found between these two groups (Table S5). Therefore, the data from 107 CDP and SDP inoculated animals were combined for further analyses.
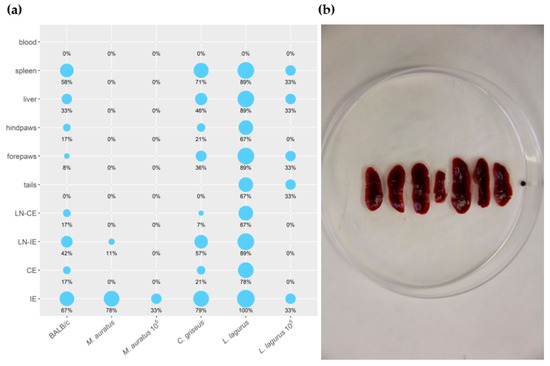
Figure 4. (a) Anatomical distribution of L. donovani in rodent species determined by qPCR. Results are presented by the balloon graph where the size of the balloon corresponds to the percentage of L. donovani infected organs from the total sum of tested organs of the same type. IE = inoculated ears; CE = contralateral ears; LN-IE = draining lymph nodes of the inoculated ears; LN-CE = draining lymph nodes of the contralateral ears. 105: Animals inoculated with 105 CDP. Animals that are not 105–labeled were inoculated with 107 CDP or SDP (b) Splenomegaly in L. lagurus infected with L. donovani in comparison with uninfected control animal (in the centre).
In M. auratus, 7/9 animals infected with 107 CDP and SDP maintained the parasite till the end of the experiment. However, Leishmania parasites were present in low numbers (<103 per sample) and remained restricted to inoculated ears or, in one specimen, draining lymph nodes of the inoculated ear (Figure 4a). In BALB/c mice, 8/12 individuals showed infection and the parasite visceralized into various tissues and organs except for the tail and blood, but again, the parasite numbers did not exceed 103. In C. griseus, Leishmania were detected in 12/14 animals and spread to all the tested tissues and organs except for the tail and blood. Contrary to M. auratus and BALB/c mice, parasite loads per sample were often higher than 103 or 104. All L. lagurus inoculated with 107 CDP and SDP were Leishmania-positive, parasites were detected in all the tested tissues except blood, and high parasite loads (>104) prevailed. Infected spleens showed distinct enlargement (Figure 4a,b).
Infectiousness to sand flies was tested from weeks 15 to 30 p.i. Phlebotomus orientalis females were allowed to feed on the whole body of anesthetized animals (Table 3). All the 514 and 207 P. orientalis females fed on BALB/c mice and M. auratus, respectively, were negative. On the contrary, for C. griseus, sand fly females became infected at week 25, and the infectiousness persisted until the end of the experiment in both 107 CDP and SDP groups. In total, 144 P. orientalis females were used for xenodiagnosis with L. lagurus. In this case, considerable differences appeared between the three groups. Specimens inoculated with 105 CDP were not infectious to the vector during the whole experiment, while in both remaining groups, sand flies became infected 25 weeks p.i. (25% of sand flies infected) and the infectiousness was still high (18.5%) or even increased (57.1%) 30 weeks p.i. in animals inoculated with SDP and 107 CDP, respectively.
Table 3. Results of xenodiagnosis experiments performed with P. orientalis in the four rodent species infected with L. donovani.
| Rodent Species | Week p.i. | No. of Animals Exposed | No. of Sand Fly Females | No. and % of Positive Females | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 105 CDP | 107 CDP | SDP |
105 CDP | 107 CDP | SDP |
105 CDP | 107 CDP |
SDP |
||
| BALB/c mice | 10 | - | 4 | 4 | - | 21 | 32 | - | 0 | 0 |
| 15 | - | 7 | 7 | - | 73 | 79 | - | 0 | 0 | |
| 20 | - | 4 | 4 | - | 55 | 76 | - | 0 | 0 | |
| 25 | - | 3 | 4 | - | 45 | 49 | - | 0 | 0 | |
| 30 | - | 2 | 4 | - | 25 | 59 | - | 0 | 0 | |
| ∑ | 219 | 295 | 0 | 0 | ||||||
| C. griseus | 10 | - | 4 | 4 | - | 11 | 7 | - | 0 | 0 |
| 15 | - | 7 | 7 | - | 22 | 33 | - | 0 | 0 | |
| 20 | - | 4 | 4 | - | 11 | 28 | - | 0 | 0 | |
| 25 | - | 4 | 4 | - | 24 | 23 | - | 1 (4.2 | 1 (4.4) | |
| 30 | - | 4 | 4 | - | 25 | 22 | - | 2 (8.0) | 1 (4.6) | |
| ∑ | 93 | 113 | 3 (3.2) | 2 (1.8) | ||||||
| M. auratus | 15 | 3 | 3 | 3 | 3 | 7 | 25 | 0 | 0 | 0 |
| 20 | 3 | 3 | 3 | 26 | 20 | NA 1 | 0 | 0 | NA | |
| 25 | 3 | 3 | 3 | 6 | 4 | 29 | 0 | 0 | 0 | |
| 30 | 3 | 3 | 6 | 13 | 14 | 60 | 0 | 0 | 0 | |
| ∑ | 48 | 45 | 114 | 0 | 0 | 0 | ||||
| L. lagurus | 15 | 3 | 3 | 3 | 9 | 17 | 4 | 0 | 0 | 0 |
| 20 | 3 | 3 | 3 | 14 | 3 | NA | 0 | 0 | NA | |
| 25 | 3 | 3 | 3 | 14 | 12 | 16 | 0 | 3 (25.0) | 4 (25.0) | |
| 30 | 3 | 3 | 6 | 14 | 14 | 27 | 0 | 8 (57.1) | 5 (18.5) | |
| ∑ | 51 | 46 | 47 | 0 | 11 (23.9) | 9 (19.1) | ||||
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671.
- Bern, C.; Maguire, J.H.; Alvar, J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl. Trop. Dis. 2008, 2, e313.
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349.
- Akilov, O.E.; Khachemoune, A.; Hasan, T. Clinical manifestations and classification of Old World cutaneous leishmaniasis. Int. J. Dermatol. 2007, 46, 132–142.
- Desjeux, P. Leishmaniasis: Public health aspects and control. Clin. Dermatol. 1996, 14, 417–423.
- Loría-Cervera, N.E.; Andrade-Narváez, J.F. Animal models for the study of leishmaniasis immunology. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 1–11.
- Guenet, J.-L.; Bonhomme, F. Wild mice: An ever-increasing contribution to a popular mammalian model. Trends Genet. 2003, 19, 24–31.
- Hommel, M.; Jaffe, C.L.; Travi, B.; Milon, G. Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann. Trop. Med. Parasitol. 1995, 89, 55–73.
- Roque, A.L.R.; Cupolillo, E.; Marchevsky, R.S.; Jansen, A.M. Thrichomys laurentius (Rodentia; Echimyidae) as a putative reservoir of Leishmania infantum and L. braziliensis: Patterns of experimental infection. PLoS Negl. Trop. Dis. 2010, 4, e589.
- Sosa-Bibiano, E.I.; Van Wynsberghe, N.R.; Canto-Lara, S.B.; Andrade-Narvaez, F.J. Preliminary study towards a novel experimental model to study localized cutaneous leishmaniasis caused by Leishmania (Leishmania) mexicana. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 165–170.
- Smyly, H.J.; Young, C.W. The experimental transmission of leishmaniasis to animals. Proc. Soc. Exp. Biol. Med. 1924, 21, 354–356.
- Young, C.W.; Smyly, H.J.; Brown, C. Experimental kala azar in a hamster, Cricetulus griseus M.Edw. Am. J. Hyg. 1926, 6, 254–275.
- Hindle, E.; Patton, W.S. Reports from the Royal Society’s Kala Azar Commission in China. No. 2.—Experiments Bearing on the Susceptibility of the Striped Hamster (Cricetulus griseus) to Leishmania of Chinese Kala Azar. Proc. R. Soc. B Biol. Sci. 1926, 374–379.
- Meleney, H.E. The Histopathology of Kala-Azar in the Hamster, Monkey, and Man. Am. J. Pathol. 1925, 1, 147–174.
- Lun, Z.R.; Wu, M.-S.; Chen, Y.-F.; Wang, J.-Y.; Zhou, X.-N.; Liao, L.-F.; Chen, J.-P.; Chow, L.M.C.; Chang, K.P. Visceral leishmaniasis in China: An endemic disease under control. Clin. Microbiol. Rev. 2015, 28, 987–1004.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms8091440
