It is impossible to describe the recent progress of our society without considering the role of polymers; however, for a broad audience, “polymer” is usually related to environmental pollution. The poor disposal and management of polymeric waste has led to an important environmental crisis, and, within polymers, plastics have attracted bad press despite being easily reprocessable. Nonetheless, there is a group of polymeric materials that is particularly more complex to reprocess, rubbers. These macromolecules are formed by irreversible crosslinked networks that give them their characteristic elastic behavior, but at the same time avoid their reprocessing. Conferring them a self-healing capacity stands out as a decisive approach for overcoming this limitation. By this mean, rubbers would be able to repair or restore their damage automatically, autonomously, or by applying an external stimulus, increasing their lifetime, and making them compatible with the circular economy model.
- self-healing materials
- self-healing rubbers
- natural rubber
- synthetic rubber
- dynamic networks
- supramolecular chemistry
1. Introduction
2. To Boldly Go Where No Material Has Gone before: Self-Healing Concepts
- Non-covalent intrinsic mechanisms, such as hydrogen bonds, ionic interactions, metal–ligand coordination, among others; and,
- Covalent intrinsic mechanisms, such as disulfide bond exchange (associative), Diels–Alder chemistry (dissociative), transesterification reactions (associative), bonds based on boron and imines chemistry (dissociative), among others.
3. Current Developments in Self-Healing Elastomers
3.1. Self-Healing Natural Rubber
|
Matrix |
Mechanism |
Healing Moieties |
Filler |
Reference |
|---|---|---|---|---|
|
NR |
Covalent intrinsic |
Diels–Alder chemistry |
Unfilled |
[55] |
|
ENR |
Non-covalent intrinsic |
Hydrogen bonds |
Unfilled |
[56] |
|
NR |
Covalent intrinsic |
Disulfide exchange |
Graphene oxide |
[57] |
|
ENR |
Combined intrinsic |
Hydrogen bonds + Transesterification reactions |
Graphene oxide |
[56] |
3.2. Self-Healing Synthetic Elastomers
|
Matrix |
Mechanism |
Healing Moieties |
Filler |
Reference |
|---|---|---|---|---|
|
SBR |
Covalent intrinsic |
Disulfide exchange |
Unfilled |
|
|
XNBR |
Non-covalent intrinsic |
Ionic interactions |
Unfilled |
[60] |
|
PUU |
Covalent intrinsic |
Disulfide exchange |
Unfilled |
|
|
Polyamide ionene |
Combined intrinsic |
Ionic interactions + Hydrogen bonds + π-π stacking |
Unfilled |
[63] |
|
Ionic elastomer |
Non-covalent intrinsic |
Ionic interactions |
Unfilled |
[64] |
|
SBR |
Covalent intrinsic |
Disulfide exchange |
GTR 1 |
|
|
SBR |
Covalent intrinsic |
Disulfide exchange |
dGTR 2 |
[66] |
|
XNBR |
Non-covalent intrinsic |
Ionic interactions |
GTR |
[60] |
|
Silicone elastomer |
Covalent intrinsic |
Thiol exchange |
Ag nanoparticles |
[67] |
1 Ground tire rubber (GTR) from end-of-life tires. 2 Devulcanized ground tire rubber (dGTR) from end-of-life tires.
4. Challenges, Perspectives, and Outlook
Until now, different matrices (natural and synthetic) with potential industrial applications have been studied. However, there is still much work to be done. Although it is true that efforts point towards the scalability of self-healing concepts in commercial applications, a comprehensive understanding of the underlying self-healing mechanisms, as well as the optimization of its conditions, is still pending. The redesign of elastomeric compounds to exhibit this capability requires the consideration of three main conditions (Figure 1):
- The construction of a dynamic but stable and robust network at service temperatures to guarantee excellent mechanical performance,
- The minimization of components that can hinder the mobility necessary to achieve healing (e.g., secondary irreversible networks) and,
- The optimization of the appropriate conditions (temporality and external stimulus) for each repair mechanism. In turn, these conditions must be compatible with the material stability, to avoid its deterioration during the healing protocols.
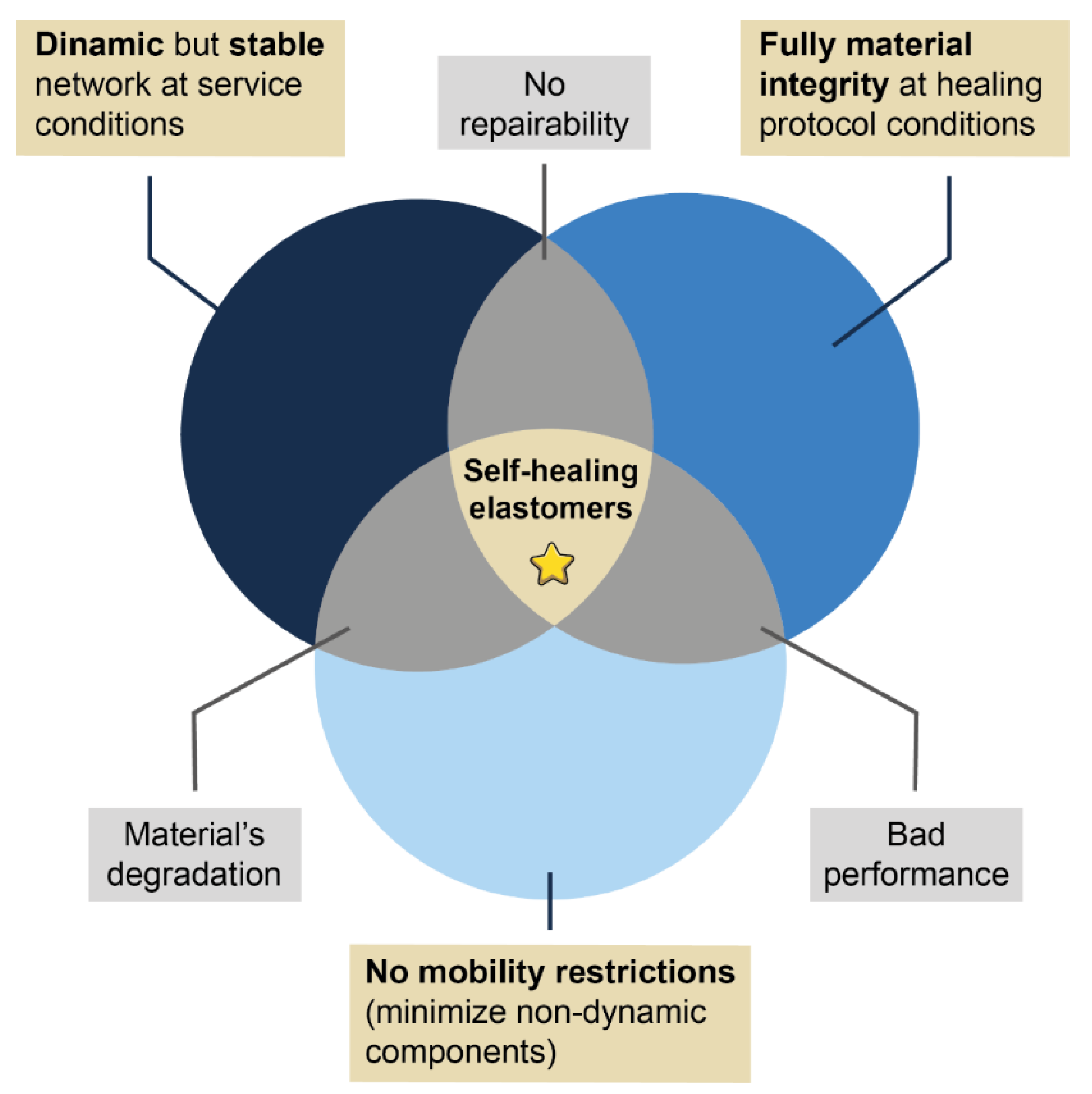 Figure 1. Venn diagram illustrating optimal healing conditions.
Figure 1. Venn diagram illustrating optimal healing conditions.
On this road it is important not overlooking the economic viability, the commercial prospect, and the corresponding life cycle assessment (LCA) that corroborates its environmental impact. When all these conditions are matched, the massive scalability of self-healing materials will be an irreversible fact, and we will have taken one of the definitive steps towards the consolidation of a truly sustainable society.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23094757
References
- Alan M. Wemyss; Chris Bowen; Cédric Plesse; Cédric Vancaeyzeele; Giao T.M. Nguyen; Frédéric Vidal; Chaoying Wan; Dynamic crosslinked rubbers for a green future: A material perspective. Materials Science and Engineering: R: Reports 2020, 141, 100561, 10.1016/j.mser.2020.100561.
- Yuxin Zhang; Zhen Zhang; Alan Matheson Wemyss; Chaoying Wan; Yongtao Liu; Pan Song; Shifeng Wang; Effective Thermal-Oxidative Reclamation of Waste Tire Rubbers for Producing High-Performance Rubber Composites. ACS Sustainable Chemistry & Engineering 2020, 8, 9079-9087, 10.1021/acssuschemeng.0c02292.
- Łukasz Zedler; Marta Przybysz-Romatowska; Józef Haponiuk; Shifeng Wang; Krzysztof Formela; Modification of Ground Tire Rubber—Promising Approach for Development of Green Composites. Journal of Composites Science 2019, 4, 2, 10.3390/jcs4010002.
- Łukasz Zedler; Xavier Colom; Javier Cañavate; Mohammad Saeb; Józef T. Haponiuk; Krzysztof Formela; Investigating the Impact of Curing System on Structure-Property Relationship of Natural Rubber Modified with Brewery By-Product and Ground Tire Rubber. Polymers 2020, 12, 545, 10.3390/polym12030545.
- Adeel Ahmad Hassan; Zhen Zhang; Krzysztof Formela; Shifeng Wang; Thermo-oxidative exfoliation of carbon black from ground tire rubber as potential reinforcement in green tires. Composites Science and Technology 2021, 214, 108991, 10.1016/j.compscitech.2021.108991.
- Krzysztof Formela; Maria Kurańska; Mateusz Barczewski; Recent Advances in Development of Waste-Based Polymer Materials: A Review. Polymers 2022, 14, 1050, 10.3390/polym14051050.
- Soumyajit Ghorai; Satyaban Bhunia; Madhusudan Roy; Debapriya De; Mechanochemical devulcanization of natural rubber vulcanizate by dual function disulfide chemicals. Polymer Degradation and Stability 2016, 129, 34-46, 10.1016/j.polymdegradstab.2016.03.024.
- Karima Aoudia; Saïd Azem; Nourredine Aït Hocine; Michel Gratton; Valeria Pettarin; Saïd Seghar; Recycling of waste tire rubber: Microwave devulcanization and incorporation in a thermoset resin. Waste Management 2017, 60, 471-481, 10.1016/j.wasman.2016.10.051.
- Fabiula D.B. de Sousa; Carlos Scuracchio; Guo-Hua Hu; Sandrine Hoppe; Devulcanization of waste tire rubber by microwaves. Polymer Degradation and Stability 2017, 138, 169-181, 10.1016/j.polymdegradstab.2017.03.008.
- Saïd Seghar; Lucia Asaro; Morena Rolland-Monnet; Nourredine Aït Hocine; Thermo-mechanical devulcanization and recycling of rubber industry waste. Resources, Conservation and Recycling 2019, 144, 180-186, 10.1016/j.resconrec.2019.01.047.
- X. Colom; J. Cañavate; K. Formela; Alireza Shadman; Mohammad Reza Saeb; Assessment of the devulcanization process of EPDM waste from roofing systems by combined thermomechanical/microwave procedures. Polymer Degradation and Stability 2020, 183, 109450, 10.1016/j.polymdegradstab.2020.109450.
- Alan M Wemyss; Christopher Ellingford; Yoshihiro Morishita; Christopher Bowen; Chaoying Wan; Dynamic Polymer Networks: A New Avenue towards Sustainable and Advanced Soft Machines. Angewandte Chemie International Edition 2021, 60, 13725-13736, 10.1002/anie.202013254.
- Nikola Bosnjak; Meredith N. Silberstein; Pathways to tough yet soft materials. Science 2021, 374, 150-151, 10.1126/science.abl6358.
- Nabarun Roy; Bernd Bruchmann; Jean-Marie Lehn; DYNAMERS: dynamic polymers as self-healing materials. Chemical Society Reviews 2015, 44, 3786-3807, 10.1039/c5cs00194c.
- Borui Zhang; Nethmi De Alwis Watuthanthrige; Shiwanka V. Wanasinghe; Saadyah Averick; Dominik Konkolewicz; Complementary Dynamic Chemistries for Multifunctional Polymeric Materials. Advanced Functional Materials 2021, 32, 2108431, 10.1002/adfm.202108431.
- Zhulu Xie; Ben-Lin Hu; Run-Wei Li; Qichun Zhang; Hydrogen Bonding in Self-Healing Elastomers. ACS Omega 2021, 6, 9319-9333, 10.1021/acsomega.1c00462.
- Qiaona Huang; Yong Liu; Sumin Li; Maiyong Zhu; Tongfan Hao; Zhiping Zhou; Yijing Nie; Blending polar rubber with polyurethane to construct self-healing rubber with multiple hydrogen bond networks. Polymer 2022, 246, 124768, 10.1016/j.polymer.2022.124768.
- Jinhui Liu; Chunlin Xiao; Jian Tang; Yudong Liu; Jing Hua; Construction of a Dual Ionic Network in Natural Rubber with High Self-Healing Efficiency through Anionic Mechanism. Industrial & Engineering Chemistry Research 2020, 59, 12755-12765, 10.1021/acs.iecr.0c01538.
- Mithun Das; Kinsuk Naskar; Development, characterization and applications of a unique self-healable elastomer: Exploring a facile metal-ligand interaction. Polymer 2021, 237, 124373, 10.1016/j.polymer.2021.124373.
- Jiarong Huang; Zhou Gong; Yukun Chen; A stretchable elastomer with recyclability and shape memory assisted self-healing capabilities based on dynamic disulfide bonds. Polymer 2022, 242, 124569, 10.1016/j.polymer.2022.124569.
- Xiangxu Chen; Matheus A. Dam; Kanji Ono; Ajit Mal; HongBin Shen; Steven R. Nutt; Kevin Sheran; Fred Wudl; A Thermally Re-mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698-1702, 10.1126/science.1065879.
- Xiangxu Chen; Fred Wudl; Ajit K. Mal; HongBin Shen; Steven R. Nutt; New Thermally Remendable Highly Cross-Linked Polymeric Materials. Macromolecules 2003, 36, 1802-1807, 10.1021/ma0210675.
- Hanze Ying; Yanfeng Zhang; Jianjun Cheng; Dynamic urea bond for the design of reversible and self-healing polymers. Nature Communications 2014, 5, 1-9, 10.1038/ncomms4218.
- Linjun Zhang; Hao Wang; Yong Zhu; Hui Xiong; Qi Wu; Shiyu Gu; Xikui Liu; Guangsu Huang; Jinrong Wu; Electron-Donating Effect Enabled Simultaneous Improvement on the Mechanical and Self-Healing Properties of Bromobutyl Rubber Ionomers. ACS Applied Materials & Interfaces 2020, 12, 53239-53246, 10.1021/acsami.0c14901.
- Tao Peng; Jiarong Huang; Zhou Gong; Jianping Ding; Yukun Chen; Multiple cross‐linked networks enhanced ENR ‐based composite with excellent self‐healing properties. Polymers for Advanced Technologies 2021, 32, 2856-2865, 10.1002/pat.5295.
- Chun-Ming Yeh; Chun-Hsiu Lin; Tzung-You Han; Yu-Ting Xiao; Yi-An Chen; Ho-Hsiu Chou; Disulfide bond and Diels–Alder reaction bond hybrid polymers with high stretchability, transparency, recyclability, and intrinsic dual healability for skin-like tactile sensing. Journal of Materials Chemistry A 2020, 9, 6109-6116, 10.1039/d0ta10135d.
- Siyang Wang; Marek W. Urban; Self‐Healable Fluorinated Copolymers Governed by Dipolar Interactions. Advanced Science 2021, 8, 2101399, 10.1002/advs.202101399.
- Xiaoyu Gao; Wenru Fan; Wei Zhu; Gao Jiuwei; Pengfang Zhang; Chen Wang; Xuewen Wang; Hesheng Xia; Zhenhua Wang; Wei Huang; et al. Tough and Healable Elastomers via Dynamic Integrated Moiety Comprising Covalent and Noncovalent Interactions. Chemistry of Materials 2022, 34, 2981-2988, 10.1021/acs.chemmater.1c03813.
- D. Yilmaz; D. Lansade; S. Lewandowski; S. Perraud; A. Llevot; S. Carlotti; Combination of permanent hydrosilylation and reversible Diels–Alder reactions for self-healing poly(dimethylsiloxane) materials with enhanced ageing properties. Materials Today Chemistry 2022, 24, 100860, 10.1016/j.mtchem.2022.100860.
- Tu Jing; Xu Heng; Xiang Guifeng; Liang Li; Pingyun Li; Xiaode Guo; Rapid self-healing and tough polyurethane based on the synergy of multi-level hydrogen and disulfide bonds for healing propellant microcracks. Materials Chemistry Frontiers 2022, 6, 1161-1171, 10.1039/d2qm00047d.
- Chee Keong Tee; Chao Wang; Ranulfo Allen; Zhenan Bao; An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nature Nanotechnology 2012, 7, 825-832, 10.1038/nnano.2012.192.
- Hong Hai Le; Frank Böhme; Aladin Sallat; Sven Wießner; Maria Auf Der Landwehr; Uta Reuter; Klaus-Werner Stöckelhuber; Gert Heinrich; Hans-Joachim Radusch; Amit Das; et al. Triggering the Self-Healing Properties of Modified Bromobutyl Rubber by Intrinsically Electrical Heating. Macromolecular Materials and Engineering 2016, 302, 1600385, 10.1002/mame.201600385.
- Kenneth Cerdan; Guy Van Assche; Peter van Puyvelde; Joost Brancart; A novel approach for the closure of large damage in self-healing elastomers using magnetic particles. Polymer 2020, 204, 122819, 10.1016/j.polymer.2020.122819.
- Yan Zhang; Hamideh Khanbareh; James Roscow; Min Pan; Chris Bowen; Chaoying Wan; Self-Healing of Materials under High Electrical Stress. Matter 2020, 3, 989-1008, 10.1016/j.matt.2020.07.020.
- Hongshuang Guo; Yi Han; Weiqiang Zhao; Jing Yang; Lei Zhang; Universally autonomous self-healing elastomer with high stretchability. Nature Communications 2020, 11, 1-9, 10.1038/s41467-020-15949-8.
- B.J. Blaiszik; S.L.B. Kramer; S.C. Olugebefola; J.S. Moore; N.R. Sottos; S.R. White; Self-Healing Polymers and Composites. Annual Review of Materials Research 2010, 40, 179-211, 10.1146/annurev-matsci-070909-104532.
- Binder, W. H. . Self-healing polymers. from principles to applications; Binder, W. H., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2014; pp. 446.
- D.G. Bekas; Kyriaki Tsirka; D. Baltzis; A.S. Paipetis; Self-healing materials: A review of advances in materials, evaluation, characterization and monitoring techniques. Composites Part B: Engineering 2016, 87, 92-119, 10.1016/j.compositesb.2015.09.057.
- Marianella Hernández Santana; Michael Den Brabander; Santiago García; Sybrand Van Der Zwaag; Routes to Make Natural Rubber Heal: A Review. Polymer Reviews 2018, 58, 585-609, 10.1080/15583724.2018.1454947.
- Saul Ismael Utrera-Barrios; Raquel Verdejo; Miguel Angel Lopez-Manchado; Marianella Hernández Santana; Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: a review. Materials Horizons 2020, 7, 2882-2902, 10.1039/d0mh00535e.
- Javier Araujo-Morera; Miguel A. López-Manchado; R. Verdejo; Marianella Hernández Santana; Unravelling the effect of healing conditions and vulcanizing additives on the healing performance of rubber networks. Polymer 2021, 238, 124399, 10.1016/j.polymer.2021.124399.
- Prasanta Kumar Behera; Subhra Mohanty; Virendra Kumar Gupta; Self-healing elastomers based on conjugated diolefins: a review. Polymer Chemistry 2021, 12, 1598-1621, 10.1039/d0py01458c.
- Fouzia Mashkoor; Sun Jin Lee; Hoon Yi; Seung Man Noh; Changyoon Jeong; Self-Healing Materials for Electronics Applications. International Journal of Molecular Sciences 2022, 23, 622, 10.3390/ijms23020622.
- Seppe Terryn; Jakob Langenbach; Ellen Roels; Joost Brancart; Camille Bakkali-Hassani; Quentin-Arthur Poutrel; Antonia Georgopoulou; Thomas George Thuruthel; Ali Safaei; Pasquale Ferrentino; et al. A review on self-healing polymers for soft robotics. Materials Today 2021, 47, 187-205, 10.1016/j.mattod.2021.01.009.
- Philippe Cordier; François Tournilhac; Corinne Soulié-Ziakovic; Ludwik Leibler; Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977-980, 10.1038/nature06669.
- Mohammad Abdul Sattar; Archita Patnaik; Design Principles of Interfacial Dynamic Bonds in Self‐Healing Materials: What are the Parameters?. Chemistry – An Asian Journal 2020, 15, 4215-4240, 10.1002/asia.202001157.
- Kohjiya, S.; Ikeda, Y.. Chemistry, manufacture, and applications of natural rubber; Kohjiya, S.; Ikeda, Y., Eds.; Woodhead Publishing: Cambridge, 2014; pp. 506.
- Xiao Men; Fan Wang; Guo-Qiang Chen; Hai-Bo Zhang; Mo Xian; Biosynthesis of Natural Rubber: Current State and Perspectives. International Journal of Molecular Sciences 2018, 20, 50, 10.3390/ijms20010050.
- S. Toki; T. Fujimaki; M. Okuyama; Strain-induced crystallization of natural rubber as detected real-time by wide-angle X-ray diffraction technique. Polymer 2000, 41, 5423-5429, 10.1016/s0032-3861(99)00724-7.
- Sureerut Amnuaypornsri; Jitladda Sakdapipanich; Shigeyuki Toki; Benjamin S. Hsiao; Naoya Ichikawa; Yasuyuki Tanaka; Strain-Induced Crystallization of Natural Rubber: Effect of Proteins and Phospholipids. Rubber Chemistry and Technology 2008, 81, 753-766, 10.5254/1.3548230.
- Javier Carretero–González; Tiberio A. Ezquerra; Sureerut Amnuaypornsri; Shigeyuki Toki; Raquel Verdejo; Alejandro Sanz; Jitladda Sakdapipanich; Benjamin S. Hsiao; Miguel A. López–Manchado; Molecular dynamics of natural rubber as revealed by dielectric spectroscopy: The role of natural cross–linking. Soft Matter 2010, 6, 3636-3642, 10.1039/c003087b.
- Sureerut Amnuaypornsri; Shigeyuki Toki; Benjamin S. Hsiao; Jitladda Sakdapipanich; The effects of endlinking network and entanglement to stress–strain relation and strain-induced crystallization of un-vulcanized and vulcanized natural rubber. Polymer 2012, 53, 3325-3330, 10.1016/j.polymer.2012.05.020.
- Shigeyuki Toki; Justin Che; Lixia Rong; Benjamin S. Hsiao; Sureerut Amnuaypornsri; Adul Nimpaiboon; Jitladda Sakdapipanich; Entanglements and Networks to Strain-Induced Crystallization and Stress–Strain Relations in Natural Rubber and Synthetic Polyisoprene at Various Temperatures. Macromolecules 2013, 46, 5238-5248, 10.1021/ma400504k.
- Toki, S. The effect of strain-induced crystallization (SIC) on the physical properties of natural rubber (NR). In Chemistry, Manufacture, and Applications of Natural Rubber; Kohjiya, S.; Ikeda, Y., Eds.; Woodhead Publishing: Cambridge, 2014; pp. 135-167.
- Paolo Tanasi; Marianella Hernández Santana; Javier Carretero-González; Raquel Verdejo; Miguel A. López-Manchado; Thermo-reversible crosslinked natural rubber: A Diels-Alder route for reuse and self-healing properties in elastomers. Polymer 2019, 175, 15-24, 10.1016/j.polymer.2019.04.059.
- Saul Utrera-Barrios; Marianella Hernández Santana; Raquel Verdejo; Miguel A. López-Manchado; Design of Rubber Composites with Autonomous Self-Healing Capability. ACS Omega 2020, 5, 1902-1910, 10.1021/acsomega.9b03516.
- Marianella Hernández; M Mar Bernal; Antonio M Grande; Nan Zhong; Sybrand Van Der Zwaag; Santiago J García; Effect of graphene content on the restoration of mechanical, electrical and thermal functionalities of a self-healing natural rubber. Smart Materials and Structures 2017, 26, 085010, 10.1088/1361-665x/aa71f5.
- Meral Yikmis; Alexander Steinbüchel; Historical and Recent Achievements in the Field of Microbial Degradation of Natural and Synthetic Rubber. Applied and Environmental Microbiology 2012, 78, 4543-4551, 10.1128/aem.00001-12.
- Marianella Hernández Santana; María Huete; Patricia Lameda; Javier Araujo; Raquel Verdejo; Miguel A. López-Manchado; Design of a new generation of sustainable SBR compounds with good trade-off between mechanical properties and self-healing ability. European Polymer Journal 2018, 106, 273-283, 10.1016/j.eurpolymj.2018.07.040.
- Saul Utrera-Barrios; Javier Araujo-Morera; Laura Pulido De Los Reyes; Reyes Verdugo Manzanares; Raquel Verdejo; Miguel Ángel López-Manchado; Marianella Hernández Santana; An effective and sustainable approach for achieving self-healing in nitrile rubber. European Polymer Journal 2020, 139, 110032, 10.1016/j.eurpolymj.2020.110032.
- Roberto Martin; Alaitz Rekondo; Alaitz Ruiz de Luzuriaga; Germán Cabañero; Hans J. Grande; Ibon Odriozola; The processability of a poly(urea-urethane) elastomer reversibly crosslinked with aromatic disulfide bridges. Journal of Materials Chemistry A 2014, 2, 5710-5715, 10.1039/c3ta14927g.
- Alaitz Rekondo; Roberto Martin; Alaitz Ruiz de Luzuriaga; Germán Cabañero; Hans J. Grande; Ibon Odriozola; Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Materials Horizons 2013, 1, 237-240, 10.1039/c3mh00061c.
- Kathryn O’Harra; Naroa Sadaba; Mikel Irigoyen; Fernando Ruipérez; Roberto Aguirresarobe; Haritz Sardon; Jason E. Bara; Nearly Perfect 3D Structures Obtained by Assembly of Printed Parts of Polyamide Ionene Self-Healing Elastomer. ACS Applied Polymer Materials 2020, 2, 4352-4359, 10.1021/acsapm.0c00799.
- Ali Aboudzadeh; Mercedes Fernandez; Maria Eugenia Muñoz; Antxon Santamaría; David Mecerreyes; Ionic Supramolecular Networks Fully Based on Chemicals Coming from Renewable Sources. Macromolecular Rapid Communications 2013, 35, 460-465, 10.1002/marc.201300732.
- Javier Araujo-Morera; Marianella Hernández Santana; Raquel Verdejo; Miguel Angel López-Manchado; Giving a Second Opportunity to Tire Waste: An Alternative Path for the Development of Sustainable Self-Healing Styrene–Butadiene Rubber Compounds Overcoming the Magic Triangle of Tires. Polymers 2019, 11, 2122, 10.3390/polym11122122.
- Luis E. Alonso Pastor; Karina C. Núñez Carrero; Javier Araujo-Morera; Marianella Hernández Santana; José María Pastor; Setting Relationships between Structure and Devulcanization of Ground Tire Rubber and Their Effect on Self-Healing Elastomers. Polymers 2021, 14, 11, 10.3390/polym14010011.
- Roberto Martín; Alaitz Rekondo; Jon Echeberria; Germán Cabañero; Hans J. Grande; Ibon Odriozola; Room temperature self-healing power of silicone elastomers having silver nanoparticles as crosslinkers. Chemical Communications 2012, 48, 8255-8257, 10.1039/c2cc32030d.
