Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Priming (also referred to as acclimation, acquired stress resistance, adaptive response, or cross-protection) is defined as an exposure of an organism to mild stress that leads to the development of a subsequent stronger and more protective response. This memory of a previously encountered stress likely provides a strong survival advantage in a rapidly shifting environment. Priming has been identified in animals, plants, fungi, and bacteria.
- fungal priming
- acclimation
- acquired stress resistance
1. Introduction
What is priming? There is growing evidence that animals, plants, bacteria, and fungi can ‘remember’ a past experience. The memory of a past event may shape or ‘prime’ their response to future external stressors, resulting in a subsequent robust response. Priming (also referred to as acclimation, acquired stress resistance, adaptive response, or cross-protection) is defined as a time-limited pre-exposure of an organism to stress that leads to an increased adaptive response to subsequent exposures [1]. The initial priming stress can be identical or different from the subsequent exposure. If the initial priming stress and the subsequent exposure are of the same nature, they are referred to as cis-priming. If priming and exposure differ, for example, heat priming followed by exposure to oxidative stress, they are termed trans-priming [1]. The duration of the memory resulting from the priming stress event can vary from days to weeks, and even in some cases, can be inherited by the offspring. Intergenerational priming occurs when the stress memory is observed in the first, stress-free offspring generation, whereas trans-generational priming is observed after more than two stress-free offspring generations [1][2][3]. Priming mechanisms are not well understood but appear to involve epigenetic, cellular, and other non-genetic mechanisms [3] (Figure 1).
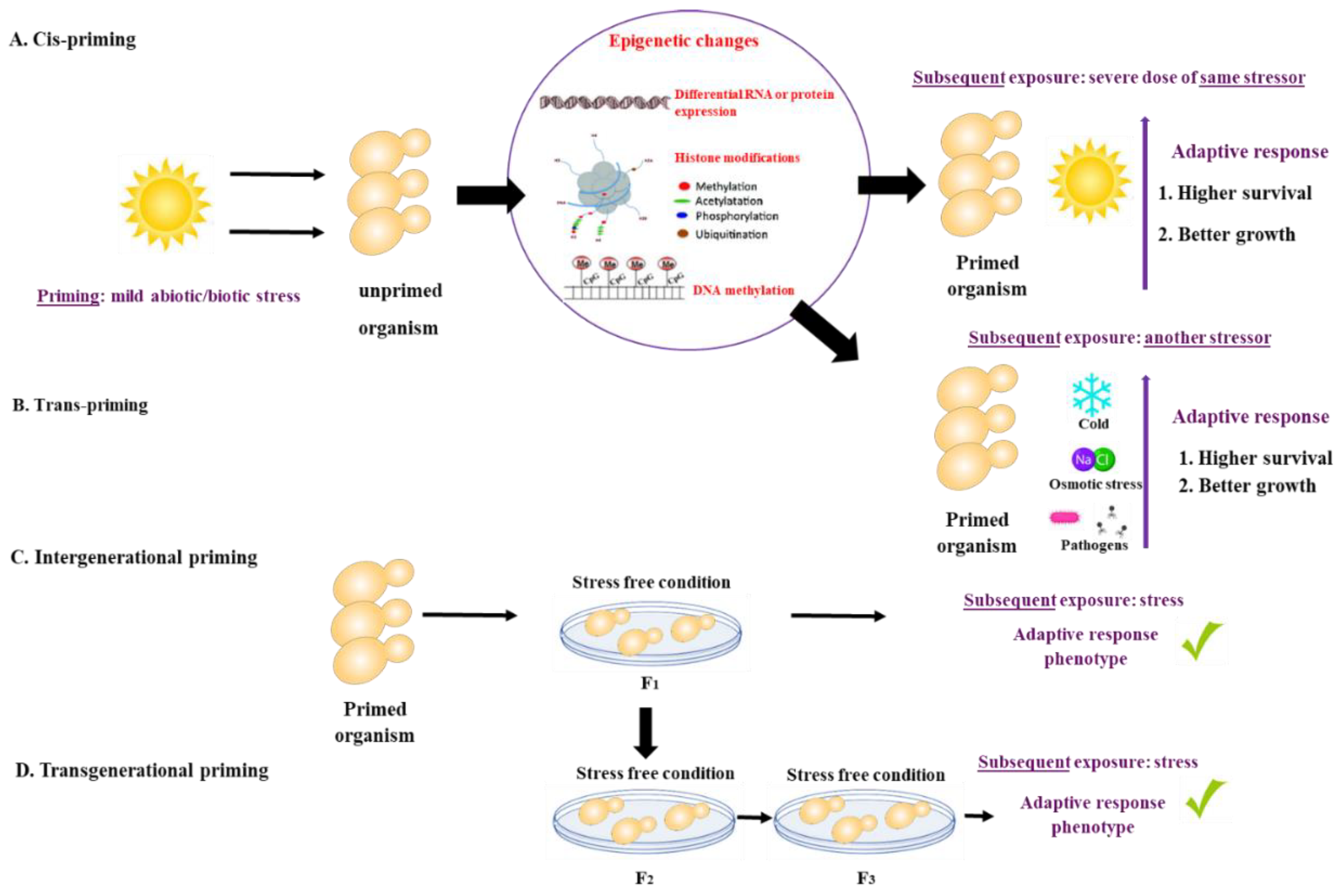 Figure 1. Priming is defined as a time-limited pre-exposure of an organism to a mild stress that leads to an increased adaptive response to subsequent exposures. (A) If the initial priming stress and the subsequent exposure are of the same nature, they are referred to as cis-priming. (B) If priming and exposure differ, they are termed trans-priming. Mechanisms of priming include differential RNA or protein expression and storage, histone modifications and DNA methylation. (C) Intergenerational priming occurs when the stress memory is observed in the first, stress-free offspring generation, while (D) trans-generational priming is observed after more than two stress-free offspring generations.
Figure 1. Priming is defined as a time-limited pre-exposure of an organism to a mild stress that leads to an increased adaptive response to subsequent exposures. (A) If the initial priming stress and the subsequent exposure are of the same nature, they are referred to as cis-priming. (B) If priming and exposure differ, they are termed trans-priming. Mechanisms of priming include differential RNA or protein expression and storage, histone modifications and DNA methylation. (C) Intergenerational priming occurs when the stress memory is observed in the first, stress-free offspring generation, while (D) trans-generational priming is observed after more than two stress-free offspring generations.Priming has been studied in vertebrates, invertebrates, plants, and bacteria [1][2][4][5][6][7][8] (Table 1). Different types of priming have been described in vertebrates and invertebrates, including immune priming [5][9] and transgenerational priming [5][6][10][11][12][13][14][15][16][17]. Priming in plants occurs primarily as a result of abiotic (e.g., drought, low and high temperatures, osmotic stress) [18][19][20][21][22] and biotic stress (e.g., fungi, viruses, bacteria, and hormones) [23][24][25], and is mainly driven by epigenetic mechanisms [2][3]. Priming in bacteria has been observed in response to both abiotic (temperature, hypoxia) [26][27] and biotic stress (antibiotics, antifungal peptides) [28][29][30][31][32], with cross resistance to biotic stress sometimes occurring as a result of abiotic stress [29][30].
Table 1. Animal, plant, and bacterial priming.
| Organism | Species | Priming Stress | Exposure Results | Mechanism | References |
|---|---|---|---|---|---|
| Mice | Mus musculus | Infection with sub-lethal concentrations of the pathogenic mold A. fumigatus | 80% survival of primed mice, rapid and severe disease onset which cleared after 3 d | Rapid phagocytosis by neutrophiles, elevated levels of the proinflammatory cytokine IL-17 | [33] |
| Mice | Mus musculus | Vaccinate mice with liposomal L-mannose protein | Higher survival rate of vaccinated mice after re-infection with C. albicans | Elevated production of polyclonal antibodies | [34] |
| Roundworms | Caenorhabditis elegans | Exposure to different stressors: heavy metals, NaCl, fasting. | Increased resistance to fatal oxidative stress which last up to F3 | Induction of the transcription factor SKN1 for the oxidative stress response | [13] |
| Insects | Galleria mellonela | Inoculation with sub lethal doses of the pathogen C.albicans | Protection against lethal doses of re-infection with same pathogen | Upregulation of antifungal genes (e.g., gallerimycin) | [35] |
| Insects | Drosophila Melanogaster | Inoculation with sublethal doses of the Pathogen S. pneumoniae | Protection against lethal doses of re-infection with same pathogen | Higher and efficient phagocytosis | [36] |
| Plants | Arabidopsis thaliana | Exposure to secreted volatiles from a damaged neighboring plant infected with M. separata larva | Protection against future herbivores | Increased Trypsin inhibitors (TI-plant defense genes) by demethylation of the TI promoter | [23] |
| Plants | Arabidopsis thaliana | Mild osmotic stress (50 mM NaCl) |
Higher tolerance to drought stress and extreme osmotic stress (80 mM NaCl) | Histone modification, and increased NaCl transporter (HKT1) induction after second exposure to salt stress | [20] |
| Plants | Arrhenatherum elatius | Dehydration periods of 16 days |
Improved photo-protection and higher biomass in second exposure to severe drought | Not known | [21] |
| Plants | Triticum aestivum (winter wheat) | Moderate drought during the vegetative growth period of the plant | Better tolerance to post-anthesis severe drought | Regulating of hormonal levels (e.g., cytokinnines) | [18] |
| Plants | Soybean seeds |
Low to mild concentrations of melatonin | Increased salt tolerance, increased drought tolerance | Upregulation of several genes involved in photosynthesis and sugar metabolism | [24] |
| Bacteria | Bacillus subtilis | Mild heat shock stress (48 °C for 15 min) | Increased tolerance against lethal heat shock stress (53 °C) | Less protein aggregation | [26] |
| Bacteria | Escherichia coli | Subinhibitory concentrations of the antibiotic Ampicillin | Increased resistance to lethal levels of ampicillin, increased resistance to lethal oxidative stress and heat shock stress. | Upregulation of genes involved in higher energy metabolism and more ribosomal production | [31] |
| Bacteria | Escherichia coli | Sublethal doses of AMPs (pexiganan and melittin) | Increased resistance to lethal doses of AMPs (pexiganan and melittin) | Higher amount of colanic-acid capsule in pexiganan-primed cells. Elevated levels of curli fimbriae in melittin-primed cells | [32] |
| Bacteria | Listeria monocytogenes | Exposure to NaCl stress | Increased resistance to the antimicrobial food preservation molecule Nisin | Increased transcript levels of LiaR-regulated genes | [29] |
2. Priming in Fungi
In fungi, priming has been most studied in the model yeast Saccharomyces cerevisiae and reflects a general homeostatic stress response [4][37]. In S. cerevisiae, the contribution of heat-shock priming to acquired stress resistance to subsequent severe stressors (trans-priming) was described in several studies [38][39][40] (Table 2). For example, S. cerevisiae primed by heat shock (37 °C, 1 h) or osmotic stress (0.7 M, 1 h) displayed tolerance to a following heat shock stress (48 °C). However, heat shock stressed cells were not tolerant against a following exposure to acute osmotic stress (1.5 M) [40]. These findings were reinforced by the study conducted by Coote et al., where priming of S. cerevisiae with several sub-lethal temperatures increased yeast thermotolerance to a higher temperature (52 °C) [38]. Yeast exposed to near freezing temperatures show increased tolerance for subsequent exposure to low temperatures and freezing. This response involves Msn2p and Msn4p transcriptional activation of the trehalose synthesizing genes TPS1 and TPS2, leading to accumulation of protective trehalose [41]. Prior exposure of S. cerevisiae to ethanol (6%) and sorbic acid (9 M) resulted in increased thermotolerance to the same subsequent heat shock stress (52 °C), suggesting that acquired thermotolerance can result from a general stress response that is not specifically caused by the same priming agent [37][38]. Heat shock or ethanol priming protects S. cerevisiae against subsequent exposure to oxidative stress, emulating the adaptive responses found in the natural habitat of this yeast [42]. These findings suggest that different priming responses in S. cerevisiae share common genetic elements. Indeed, Hsp104p, responsible for disassembling protein aggregates, was strongly induced following priming by both heat and ethanol stress. Similarly, the disaccharide trehalose is strongly induced by heat, ethanol, and oxidative stress priming, functioning as a chemical protein-folding chaperone, free radical scavenger, and stabilizer of phospholipid membranes [37]. Mutational and transcriptome analysis revealed that the “general stress” transcription factors Msn2p and Msn4p [43], as well as the transcription factor Mga2 [44], involved in fatty acid biosynthesis, ergosterol biosynthesis, and the response to hypoxia, are required for heat, salt, and oxidative stress priming. Likewise, regulatory cross-talk between the transcription factors Msn2p, Msn4p, and Pdr3p was seen following priming by heat, organic acids, and osmotic stress [37]. Priming in yeast requires nascent protein synthesis during the pre-exposure step [43] and can persist for up to five generations, suggesting an epigenetic mechanism [39].
Table 2. Fungal priming.
| Species | Priming Stress | Exposure Result | Mechanism | References |
|---|---|---|---|---|
| Saccharomyces cerevisiae | Heat stress (37 °C, 1 h) or osmotic stress (0.7 M, 1 h) | Better tolerance to severe heat shock (47 °C) | Induction of GPDH in osmotic stressed cells, but not in heat-shock stressed cells. Mechanism unknown. | [40] |
| Saccharomyces cerevisiae | Exposure to sub-lethal temperatures | Increased thermotolerance | Mechanism involves the ATPase proton pump | [38] |
| Saccharomyces cerevisiae | Oxidative stress (H2O2), sub-lethal ethanol stress, cold stress | Barotolerence or high hydrostatic pressure tolerance (HHP) | Upregulation of genes involved in oxidative stress defense response, cell membrane changes | [45] |
| Saccharomyces cerevisiae | Exposure to several mild stressors (acute heat, NaCl, oxidative stress, ethanol) | Resistance to same stressor * (e.g., p.heat-s.heat), resistance to some subsequent stressors (p.NaCl-s.NaCl and H2O2) | Induction of transcription factors Msn2p and/or Msn4p | [43] |
| Saccharomyces cerevisiae | Salt (NaCl) stress | Tolerance to severe oxidative stress (H2O2) | A role for the nuclear pore component Nup42p | [39] |
| Candida albicans | Mild heat, osmotic or oxidative stress | Heat stressed cells exhibited tolerance to a strong oxidative stress | Slightly increased in HSPs levels (heat shock protein) | [46] |
| Candida albicans | Different concentrations of glucose, low, mild and high | Increased resistance to the antifungal miconazole, increased resistance to osmotic stress and oxidative stress | Upregulation of genes involved in drug resistance, induction of osmotic stress related genes, | [47] |
| Metarhizium anisopliae | Sub-lethal concentrations of heat stress, oxidative stress, osmotic stress and nutritive stress | Increased resistance of p.nutritive and p.oxidative -s.UV-B and s.heat. Increased resistance of p.heat–s.UV-B and heat |
Partially established, increased levels of sugars (trehalose and mannitol) | [48] |
| Agaricus bisporus | Application of exogenous riboflavin/vitamin B2 | Increased drought resistance | Not established, but changes in transcripts levels was observed | [49] |
| Penicillium chrysogenum | Mild drought stress | Increased resistance to severe drought | Higher β-glucosidase and respiratory activity | [50] |
| Rhizopus arrhyzus | Exposure to sub-lethal concentrations of voriconazole or isavuconazole | In vivo hypervirulence observed by lower survival rate of fruit flies (D. melanogaster) | Unknown | [51] |
| Rhizopus arrhyzus | Tornadic shear stress | Hypervirulence in D. melanogaster in vivo model | Secreted metabolites and calcineurin-signaling pathway (not fully characterized) | [52] |
| Aspergillus fumigatus | Mild heat stress (37 or 45 °C) | Increased resistance to both oxidative stress and severe heat stress (60 °C) | Increased sugar content (trehalose) | [53] |
| Aspergillus fumigatus | Different environmental stressors: minimal medium, 50 °C, NaCl, +Fe and -Zn | Increased pathogenicity in D. melanogaster in vivo model | Not established | [54] |
| Aspergillus fumigatus | Osmotic stress (0.5M NaCl or KCl) Zinc-starved stress, cold (4 °C) or heat (42 °C induced stress | Increased tolerance to oxidative stress, or zinc-stress | Multiple changes in genes transcript levels: ZapA (osmotic stress), Hsp70 and ZapA (heat and zinc-starves stress), ergosterol pathway (osmotic stress), gliotoxin secretion (Zinc-starved stress) | [55] |
* p = priming stress; s = subsequent stress.
In contrast to the “general stress” response in S. cerevisiae, the pathogenic yeast Candida albicans does not show strong cross-protection to a subsequent stressor which is different from the pretreatment [56]. For example, pretreatment of C. albicans with mild heat shock only slightly increased its resistance to oxidative stress, and mild stress, induced by osmotic or oxidative stress, did not improve the survival rate of the yeast when a subsequent heat shock stress was applied [46] (Table 2).
Although the general stress response and adaptation to several specific stressors differ between S. cerevisiae and C. albicans [56], both fungi were highly sensitive to glucose levels, a fact which may affect their response to a following stress stimulus. Rodaki et al. demonstrated that in C. albicans, stress-related genes such as TPS1, TPS2, and TPS3 involved in the biosynthesis of trehalose were upregulated upon priming with low concentrations of glucose [47]. This upregulation helped the fungus better cope with subsequent oxidative and cationic stress and also contributed to azole antifungal resistance, indicating that cross-protection occurs in C. albicans. Interestingly, glucose treatment in S. cerevisiae leads to down-regulation of stress response genes [47]. The differences between S. cerevisiae and C. albicans cross-resistance were further investigated by Enjalbert et al., who revealed that C. albicans has a core stress response, but one that contains a smaller subset of genes [57], including the stress-activated protein Hog1 and the transcription factor Cap1 which have diverged significantly from S. cerevisiae [56][57][58]. In addition, homologs of the S. cerevisiae transcription factors Msn2 and Msn4, involved in regulating its core stress response, do not play equivalent roles in C. albicans [59]. These molecular differences between the two yeasts, resulting from their radically different lifestyles, may explain their different priming responses and cross-resistance to various stressors.
3. Priming in the Filamentous Fungi
In filamentous fungi, the phenomenon of priming has been understudied and remains generally descriptive [4] (Table 2). Priming has been described following exposure to several exogenous stresses, such as insect grazing, drugs, shear stress, or high temperatures [51][52][60]. Temperature priming was demonstrated in several species of Ascomycetes and Mucorales soil fungi, indicating that priming phenomena are likely widespread [61]. The duration of priming memory was tested in a recent study in two filamentous fungi. Neurospora crassa and Penicillium chrysogenum were primed by drought, and the priming response duration or memory was measured [50]. Both fungi were exposed to mild drought, then allowed to recover for 1, 7, or 14 d, then triggered again with severe drought. Following this stress, the P. chrysogenum-primed conidia showed improved growth and metabolic activity compared to unprimed conidia for up to 14 days, whereas in N. crassa, no priming responses were observed. This suggests that unlike in yeast, the priming-induced memory in some filamentous fungi can last for weeks.
The filamentous fungus Rhizopus arrhizus, which belongs to the Mucorales division of fungi and is known to cause life-threatening infection in immunocompromised patients, was also studied for priming. R. arrhizus spores were primed for 30 min by magnetic stirring to mimic tornadic shear stress. Then, the tornadic shear-primed spores were injected into Drosophila melanogaster fruit flies, and the survival rate of the flies was determined. The flies injected with shear-primed spores exhibited significantly higher mortality than those injected with unprimed spores. The priming effect observed in this fungus was attributed to both secreted metabolites from the fungus and activation of the calcineurin-signaling pathway [52]. Similarly, increased R. arrhizus virulence in D. melanogaster was also seen after priming with the medical triazole voriconazole [51].
In summary, little is known about the priming responses of filamentous fungi to osmotic, nutritive, cold, and other abiotic and biotic stressors. Moreover, priming may affect fungi not only at the individual level but also at the community level, since in nature the growth of isolated filamentous fungi is rare [62]. Therefore, understanding priming may also contribute to better understanding the dynamics between filamentous fungi.
4. Priming in Aspergilli
Several recent studies have described interesting examples of priming in the Aspergilli (Table 2). Doll et al. studied the priming effect of fungivore grazing by Folsomia candida on Aspergillus nidulans [63]. Fungivore-exposed colonies of A. nidulans produced significantly higher amounts of toxic secondary metabolites and higher ascospore production relative to unchallenged fungi.
Hagiwara et al. investigated the effects of temperature during conidiation on the stress resistance of A. fumigatus conidia [53]. Conidia generated under elevated temperature priming showed a higher tolerance for heat, oxidative stress, and UV radiation. This was accompanied by increased trehalose levels, which may protect against these stresses. In another study, A. fumigatus was primed under nine different environmental conditions [54]. Each condition generated conidia with different germination and growth rates. For example, conidia generated under osmotic stress priming grew faster under various conditions. In contrast, conidia primed under metal deficiency exhibited slower germination and growth. Priming at 50 °C generated larger conidia and killed G. mellonella larvae faster. The priming mechanism underlying the hypervirulence of these conidia remains unknown.
In a landmark study, Wang et al. showed that conidia of the two filamentous fungi, A. fumigatus and A. nidulans remained transcriptionally active as long as they remained attached to the conidiophore. This is a surprising finding, as it was previously believed that mature conidia attached to the conidiophore were transcriptionally dormant [55]. Wang et al. showed that attached conidia exposed to various stresses underwent specific transcriptional changes and were more resistant to subsequent exposure to the same (cis-priming) or different (trans-priming) stresses. For example, priming with NaCl, generated conidia with higher levels of ergosterol pathway transcripts and increased tolerance to azole antifungals. Interestingly, it also increased the virulence of the conidia, following infection into Galleria mellonella moth larvae. Priming under conditions of zinc starvation generated conidia with high levels of gliotoxin biosynthesis gene transcripts. These conidia were more resistant to gliotoxin exposure. Conidial priming could explain the heterogeneity of responses seen within a population of spores exposed to stressors [64]. Each conidium contains a slightly different composition of stored proteins and RNAs based on its location in the conidial chain, which could affect its subsequent response.
In summary, these recent studies have shown that the growth of A. fumigatus and A. nidulans under stressful environmental conditions can enhance the stress resistance and virulence of the asexual spores (conidia) that they produce. This conidial priming response occurs by transcriptional modulation within the attached conidia that “prepares” them for the subsequent stress [54][55]. It will be of great interest to see if this phenomenon exists in other sporogenic fungi, including other human and plant pathogens. Recently, researchers showed that when A. fumigatus is grown in the presence of subinhibitory concentrations of agricultural triazoles, the conidia it generates are primed and partially protected against subsequent exposure to the medical triazole voriconazole (Harish et al. submitted). Furthermore, these primed conidia develop stable voriconazole resistance at higher rates compared to unprimed conidia. There is already strong evidence that agricultural triazole antifungals generate stable genetic resistance in environmental A. fumigatus, through mutations in cyp51A that also confer resistance to medical triazoles [65][66]. Therefore, the possibility that agricultural triazoles sprayed on fields at sub-lethal concentrations can prime A. fumigatus conidia towards subsequent exposure with medical triazoles or lead to increased resistance to agricultural triazole fungicides in the field is concerning. Further studies performed in an agricultural setting need to be performed to address these concerns.
This entry is adapted from the peer-reviewed paper 10.3390/jof8050448
References
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Baurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. Camb. Philos. Soc. 2015, 91, 1118–1133.
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761.
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124.
- Andrade-Linares, D.R.; Lehmann, A.; Rillig, M.C. Microbial stress priming: A meta-analysis. Environ. Microbiol. 2016, 18, 1277–1288.
- Heard, E.; Martienssen, R.A. Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell 2014, 157, 95–109.
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973.
- Milutinović, B.; Peuß, R.; Ferro, K.; Kurtz, J. Immune priming in arthropods: An update focusing on the red flour beetle. Zoology 2016, 119, 254–261.
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: The secret weapon of the insect world. Virulence 2020, 11, 238–246.
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe 2011, 9, 355–361.
- Dubuffet, A.; Zanchi, C.; Boutet, G.; Moreau, J.; Moret, Y.; Teixeira, M. Data from: Trans-generational immune priming protects the eggs only against gram-positive bacteria in the mealworm beetle. PLoS Pathog. 2015, 11, e1005178.
- Gegner, J.; Baudach, A.; Mukherjee, K.; Halitschke, R.; Vogel, H.; Vilcinskas, A. Epigenetic Mechanisms Are Involved in Sex-Specific Trans-Generational Immune Priming in the Lepidopteran Model Host Manduca sexta. Front. Physiol. 2019, 10, 137.
- Greer, E.L.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.A.; Shi, Y.; Brunet, A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 2011, 479, 365–371.
- Kishimoto, S.; Uno, M.; Okabe, E.; Nono, M.; Nishida, E. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nat. Commun. 2017, 8, 14031.
- Lisch, D. Regulation of transposable elements in maize. Curr. Opin. Plant Biol. 2012, 15, 511–516.
- Lumey, L.H.; Stein, A.D.; Kahn, H.S.; Romijn, J.A. Lipid profiles in middle-aged men and women after famine exposure during gestation: The Dutch Hunger Winter Families Study. Am. J. Clin. Nutr. 2009, 89, 1737–1743.
- Moret, Y.; Siva-Jothy, M.T. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B Boil. Sci. 2003, 270, 2475–2480.
- Rechavi, O.; Minevich, G.; Hobert, O. Transgenerational Inheritance of an Acquired Small RNA-Based Antiviral Response in C. elegans. Cell 2011, 147, 1248–1256.
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615.
- Liu, H.; Able, A.J.; Able, J.A. Small RNAs and their targets are associated with the transgenerational effects of water-deficit stress in durum wheat. Sci. Rep. 2021, 11, 3613.
- Sani, E.; Herzyk, P.; Perrella, G.; Colot, V.; Amtmann, A. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biol. 2013, 14, R59.
- Walter, J.N.Y.; Hein, R.; Rascher, U.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 2011, 71, 34–40.
- Zheng, X.; Chen, L.; Li, M.; Lou, Q.; Xia, H.; Wang, P.; Li, T.; Liu, H.; Luo, L. Transgenerational Variations in DNA Methylation Induced by Drought Stress in Two Rice Varieties with Distinguished Difference to Drought Resistance. PLoS ONE 2013, 8, e80253.
- Ali, M.; Sugimoto, K.; Ramadan, A.; Arimura, G.-I. Memory of plant communications for priming anti-herbivore responses. Sci. Rep. 2013, 3, 1872.
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707.
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Wu, S.; Ma, X.; Li, C.; Zou, Y.; et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434.
- Runde, S.; Molière, N.; Heinz, A.; Maisonneuve, E.; Janczikowski, A.; Elsholz, A.K.W.; Gerth, U.; Hecker, M.; Turgay, K. The role of thiol oxidative stress response in heat-induced protein aggregate formation during thermotolerance in Bacillus subtilis. Mol. Microbiol. 2014, 91, 1036–1052.
- Tagkopoulos, I.; Liu, Y.-C.; Tavazoie, S. Predictive Behavior within Microbial Genetic Networks. Science 2008, 320, 1313–1317.
- Bergholz, T.M.; Bowen, B.; Wiedmann, M.; Boor, K.J. Listeria monocytogenes Shows Temperature-Dependent and -Independent Responses to Salt Stress, Including Responses That Induce Cross-Protection against Other Stresses. Appl. Environ. Microbiol. 2012, 78, 2602–2612.
- Bergholz, T.M.; Tang, S.; Wiedmann, M.; Boor, K.J. Nisin Resistance of Listeria monocytogenes Is Increased by Exposure to Salt Stress and Is Mediated via LiaR. Appl. Environ. Microbiol. 2013, 79, 5682–5688.
- Fleitas, O.; Franco, O.L. Induced Bacterial Cross-Resistance toward Host Antimicrobial Peptides: A Worrying Phenomenon. Front. Microbiol. 2016, 7, 381.
- Mathieu, A.; Fleurier, S.; Frenoy, A.; Dairou, J.; Bredeche, M.-F.; Sanchez-Vizuete, P.; Song, X.; Matic, I. Discovery and Function of a General Core Hormetic Stress Response in E. coli Induced by Sublethal Concentrations of Antibiotics. Cell Rep. 2016, 17, 46–57.
- Rodríguez-Rojas, A.; Baeder, D.Y.; Johnston, P.; Regoes, R.R.; Rolff, J. Bacteria primed by antimicrobial peptides develop tolerance and persist. PLoS Pathog. 2021, 17, e1009443.
- Savers, A.; Rasid, O.; Parlato, M.; Brock, M.; Jouvion, G.; Ryffel, B.; Cavaillon, J.-M.; Eberl, G.; Ibrahim-Granet, O. Infection-Mediated Priming of Phagocytes Protects against Lethal Secondary Aspergillus fumigatus Challenge. PLoS ONE 2016, 11, e0153829.
- Han, Y.; Cutler, J.E. Antibody response that protects against disseminated candidiasis. Infect. Immun. 1995, 63, 2714–2719.
- Bergin, D.; Murphy, L.; Keenan, J.; Clynes, M.; Kavanagh, K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006, 8, 2105–2112.
- Pham, L.N.; Dionne, M.S.; Shirasu-Hiza, M.; Schneider, D.S. A Specific Primed Immune Response in Drosophila Is Dependent on Phagocytes. PLoS Pathog. 2007, 3, e26.
- Święciło, A. Cross-stress resistance in Saccharomyces cerevisiae yeast—New insight into an old phenomenon. Cell Stress Chaperones 2016, 21, 187–200.
- Coote, P.J.; Cole, M.B.; Jones, M.V. Induction of increased thermotolerance in Saccharomyces cerevisiae may be triggered by a mechanism involving intracellular pH. J. Gen. Microbiol. 1991, 137, 1701–1708.
- Guan, Q.; Haroon, S.; Bravo, D.G.; Will, J.L.; Gasch, A.P. Cellular Memory of Acquired Stress Resistance in Saccharomyces cerevisiae. Genetics 2012, 192, 495–505.
- Trollmo, C.; André, L.; Blomberg, A.; Adler, L. Physiological overlap between osmotolerance and thermotolerance in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1988, 56, 321–325.
- Kandror, O.; Bretschneider, N.; Kreydin, E.; Cavalieri, D.; Goldberg, A.L. Yeast Adapt to Near-Freezing Temperatures by STRE/Msn2,4-Dependent Induction of Trehalose Synthesis and Certain Molecular Chaperones. Mol. Cell 2004, 13, 771–781.
- Mitchell, A.; Romano, G.H.; Groisman, B.; Yona, A.; Dekel, E.; Kupiec, M.; Dahan, O.; Pilpel, Y. Adaptive prediction of environmental changes by microorganisms. Nature 2009, 460, 220–224.
- Berry, D.B.; Gasch, A.P. Stress-activated Genomic Expression Changes Serve a Preparative Role for Impending Stress in Yeast. Mol. Biol. Cell 2008, 19, 4580–4587.
- Kelley, R.; Ideker, T. Genome-Wide Fitness and Expression Profiling Implicate Mga2 in Adaptation to Hydrogen Peroxide. PLoS Genet. 2009, 5, e1000488.
- Palhano, F.L.; Orlando, M.T.; Fernandes, P.M. Induction of baroresistance by hydrogen peroxide, ethanol and cold-shock in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2004, 233, 139–145.
- Enjalbert, B.; Nantel, A.; Whiteway, M. Stress-induced Gene Expression in Candida albicans: Absence of a General Stress Response. Mol. Biol. Cell 2003, 14, 1460–1467.
- Rodaki, A.; Bohovych, I.M.; Enjalbert, B.; Young, T.; Odds, F.C.; Gow, N.A.; Brown, A.J. Glucose Promotes Stress Resistance in the Fungal Pathogen Candida albicans. Mol. Biol. Cell 2009, 20, 4845–4855.
- Rangel, D.E.; Anderson, A.J.; Roberts, D.W. Evaluating physical and nutritional stress during mycelial growth as inducers of tolerance to heat and UV-B radiation in Metarhizium anisopliae conidia. Mycol. Res. 2008, 112, 1362–1372.
- Guhr, A.; Horn, M.A.; Weig, A.R. Vitamin B2 (riboflavin) increases drought tolerance of Agaricus bisporus. Mycologia 2017, 109, 860–873.
- Guhr, A.; Kircher, S. Drought-Induced Stress Priming in Two Distinct Filamentous Saprotrophic Fungi. Microb. Ecol. 2020, 80, 27–33.
- Wurster, S.; Lewis, R.E.; Albert, N.D.; Kontoyiannis, D.P. Preexposure to Isavuconazole Increases the Virulence of Mucorales but Not Aspergillus fumigatus in a Drosophila melanogaster Infection Model. Antimicrob. Agents Chemother. 2019, 63, e01896-18.
- Wurster, S.; Tatara, A.M.; Albert, N.D.; Ibrahim, A.S.; Heitman, J.; Lee, S.C.; Shetty, A.C.; McCracken, C.; Graf, K.T.; Mikos, A.G.; et al. Tornadic Shear Stress Induces a Transient, Calcineurin-Dependent Hypervirulent Phenotype in Mucorales Molds. mBio 2020, 11, e01414-20.
- Hagiwara, D.; Sakai, K.; Suzuki, S.; Umemura, M.; Nogawa, T.; Kato, N.; Osada, H.; Watanabe, A.; Kawamoto, S.; Gonoi, T.; et al. Temperature during conidiation affects stress tolerance, pigmentation, and trypacidin accumulation in the conidia of the airborne pathogen Aspergillus fumigatus. PLoS ONE 2017, 12, e0177050.
- Kang, S.E.; Celia, B.N.; Bensasson, D.; Momany, M. Sporulation environment drives phenotypic variation in the pathogen Aspergillus fumigatus. G3 2021, 11, jkab208. 1-7.
- Wang, F.; Sethiya, P.; Hu, X.; Guo, S.; Chen, Y.; Li, A.; Tan, K.; Wong, K.H. Transcription in fungal conidia before dormancy produces phenotypically variable conidia that maximize survival in different environments. Nat. Microbiol. 2021, 6, 1066–1081.
- Brown, A.J.; Budge, S.; Kaloriti, D.; Tillmann, A.; Jacobsen, M.D.; Yin, Z.; Ene, I.V.; Bohovych, I.; Sandai, D.; Kastora, S.; et al. Stress adaptation in a pathogenic fungus. J. Exp. Biol. 2009, 217, 144–155.
- Enjalbert, B.; Smith, D.A.; Cornell, M.J.; Alam, I.; Nicholls, S.; Brown, A.J.; Quinn, J. Role of the Hog1 Stress-activated Protein Kinase in the Global Transcriptional Response to Stress in the Fungal Pathogen Candida albicans. Mol. Biol. Cell 2006, 17, 1018–1032.
- Leach, M.D.; Budge, S.; Walker, L.; Munro, C.; Cowen, L.E.; Brown, A.J.P. Hsp90 Orchestrates Transcriptional Regulation by Hsf1 and Cell Wall Remodelling by MAPK Signalling during Thermal Adaptation in a Pathogenic Yeast. PLoS Pathog. 2012, 8, e1003069.
- Nicholls, S.; Straffon, M.; Enjalbert, B.; Nantel, A.; Macaskill, S.; Whiteway, M.; Brown, A.J.P. Msn2- and Msn4-Like Transcription Factors Play No Obvious Roles in the Stress Responses of the Fungal Pathogen Candida albicans. Eukaryot. Cell 2004, 3, 1111–1123.
- Caballero Ortiz, S.; Trienens, M.; Rohlfs, M. Induced fungal resistance to insect grazing: Reciprocal fitness consequences and fungal gene expression in the Drosophila-Aspergillus model system. PLoS ONE 2013, 8, e74951.
- Andrade-Linares, D.R.; Veresoglou, S.D.; Rillig, M.C. Temperature priming and memory in soil filamentous fungi. Fungal Ecol. 2016, 21, 10–15.
- Bahram, M.; Netherway, T. Fungi as mediators linking organisms and ecosystems. FEMS Microbiol. Rev. 2021, 46, fuab058. 1-16.
- Döll, K.; Chatterjee, S.; Scheu, S.; Karlovsky, P.; Rohlfs, M. Fungal metabolic plasticity and sexual development mediate induced resistance to arthropod fungivory. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131219.
- Bleichrodt, R.-J.; Foster, P.; Howell, G.; Latgé, J.-P.; Read, N.D. Cell Wall Composition Heterogeneity between Single Cells in Aspergillus fumigatus Leads to Heterogeneous Behavior during Antifungal Treatment and Phagocytosis. mBio 2020, 11, e03015-19.
- Berger, S.; el Chazli, Y.; Babu, A.F.; Coste, A.T. Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024.
- Snelders, E.; Camps, S.M.T.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.; Van Der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.G.; Verweij, P.E. Triazole Fungicides can Induce Cross-Resistance to Medical Triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801.
This entry is offline, you can click here to edit this entry!
