Two types of radiation can be distinguished: non-ionizing and ionizing radiation; the first radiation comprises optical radiation and electromagnetic fields [1]. The optical category is divided into ultraviolet, visible, and infra-red subcategories, while the electromagnetic one can be subdivided depending on the radiofrequency [1][2]. Non-ionizing radiation can be obtained from several natural sources like the Sun and lighting, or man-made sources as those used in industrial/medical applications and wireless communications [1]. On average, a person receives approximately 2.4 mGy of natural-originated radiation every year, and this can vary according to their geographical location, for instance, countries such as Brazil, India, and China are among those with high levels of terrestrial radiation [3][4]. Ionizing radiation comprises electrically charged particles (ions), positive ones such as alpha particles and negative ones such as electrons [2]. This radiation was found after the discovery of X-rays in 1895 [5].
According to the Advisory Committee on Human Radiation Experiments alpha, beta, and gamma/X-ray radiation are the most known ionizing radiation; alpha particles are formed by two neutrons and two protons from the nucleus of the atom during the decrease of the atomic mass number and reduction of the atomic number; it results from the radioactive decay of heavy elements such as plutonium, radium, or thorium and its weight does not allow them to travel far away, being stopped by a piece of paper, and although these particles cannot pass through paper or our skin, if they are released into the body from a radioactive source, they can affect cells in our body, damaging the cells and the DNA [6]. Unlike alpha particles, beta particles are negatively charged when emitted during radioactive decay [7]. Even though these particles can reach longer distances, they can be blocked by a thin layer of substance; however, if they are swallowed or inhaled the damage can be high as that caused by alpha particles [7]. Regarding gamma rays, this radiation is frequently emitted during the radioactive decay along with alpha or beta particles, they are high-energy photons. Gamma radiation is high-energy electromagnetic radiation emitted along with alpha and beta particles during radioactive decay. Gamma particles are pure energy. Different from alpha and beta particles, gamma particles can easily penetrate the skin and cause serious tissue and DNA damage [7]. Similarly, X-rays are also pure energy but are emitted from parts of the atom different from the nucleus. This radiation is widely used in the medical field and industrial processes [7][6]. Other types of ionizing radiation can be found, such as cosmic radiation that penetrates our atmosphere and comprises mainly protons, alpha particles, and heavier atomic nuclei [8].
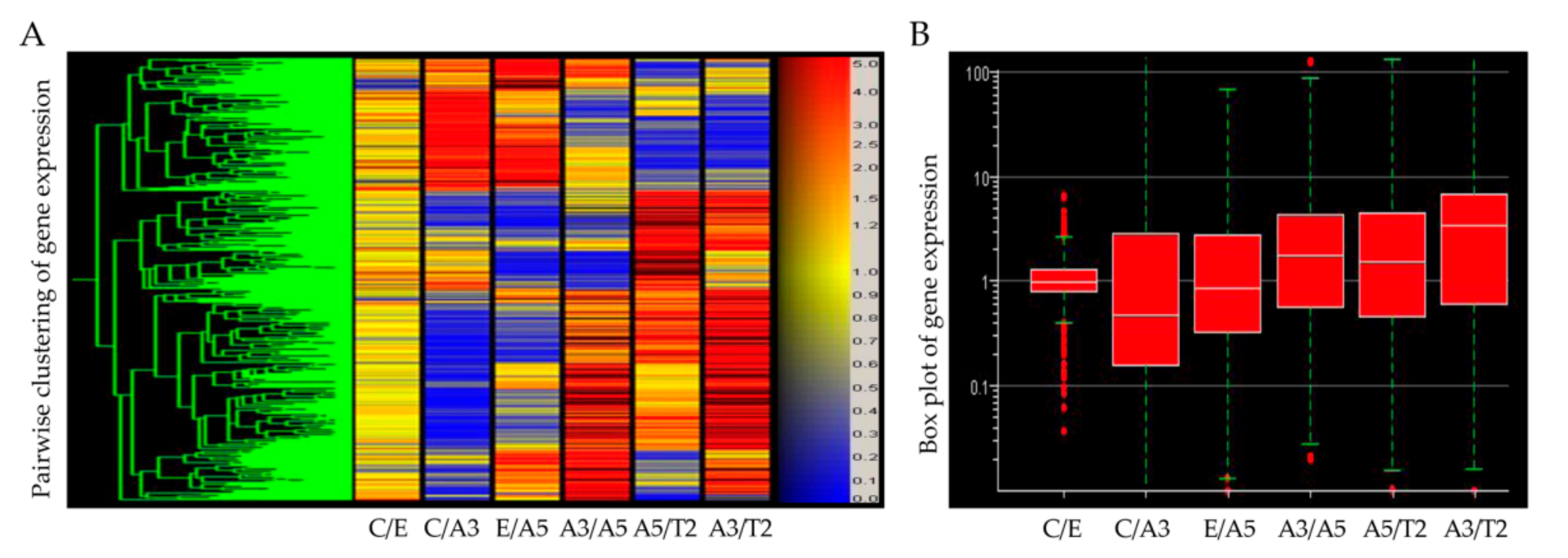
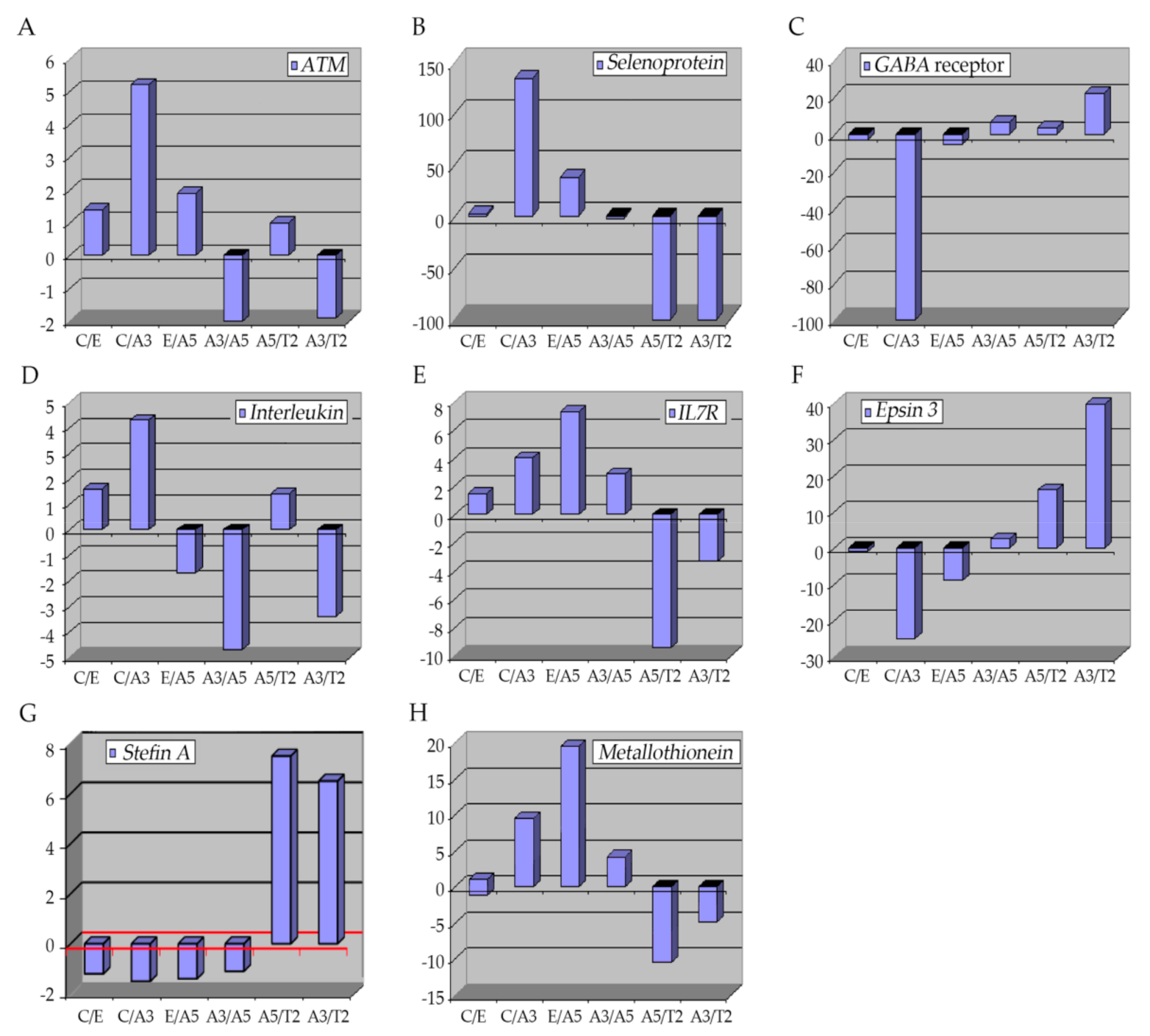
Figure 3. Graphs show the profiling of differentially expressed genes obtained through an Affymetrix array U133A data comparing these genes: (A) ATM, (B) selenoproteins, (C) GABA receptor, (D,E) interleukins, (F) Epsin 3, (G) Stefin A (CSTA), and (H) Metallothioneins in Heatmap of Affymetrix array (U133A) data that allows comparing the following cell lines: MCF-10F (C)/Estrogen (E); C/Alpha3 (A3); E/Alpha5 (A5); A3/Alpha5; A5/Tumor2 (T2) and A3/T2. All graphs were obtained from a Cluster-dendrogram repository of gene expression from our laboratory for this review.
2.1. The Ataxia-Telangiectasia Mutated Gene
The ataxia-telangiectasia mutated gene (
ATM) encodes for a 350 kDa protein serine/threonine kinase, key in the DNA-damage response elements since it can detect double-strand breaks (DSBs), fundamental in the cell-cycle checkpoint controlling
[17][18][19][20]. The
ATM gene has 66 exons with approximately 150 kb of genomic expansion
[18]. Mutations of the
ATM gene can explain ataxia telangiectasia (AT), a rare neurodegenerative disease, which is manifested clinically by skin and ocular telangiectasia, immunological deficiency, neuronal deficiency, sino-pulmonary infections, cellular sensitivity to ionizing radiation, and predisposition to cancer
[21][22][23][24][25][26][27]. However, it has been estimated that approximately 2% of the adult population presents the heterozygosity for an
ATM variant
[28][29][30]. Even though this group exhibits no phenotypical abnormalities, it has been reported that
ATM heterozygotes have a high risk of developing breast cancer, with about a 5-fold increase compared with the general population
[23][31][32][33][34][35][36]. Normally,
ATM expression is down-regulated in breast cancer tissues
[36]. Locally advanced breast tumors have shown a reduced expression of
ATM by epigenetic silencing
[37].
2.2. Selenoproteins (SEPP1)
These are metalloproteins with certain characteristics that allow them to have a high affinity to metal; they present a specific amino acid, seleno-cysteine (Se-Cys)
[38]. There are 25 types of selenoproteins in humans with this characteristic
[39][40]. Selenoproteins are involved in several cellular processes related to metastasis, comprising cell adhesion, matrix degradation, migration, invasion, and proliferation
[41]. Among these processes, roles in redox balance and calcium equilibrium have been described
[41], but the metabolism of these proteins can also modify signaling pathways in cancer cells
[42].
Glutathione peroxidases (GPXs), are selenoproteins that are responsible for the protection of tissues against reductions derived from the action of ROS such as those produced during ionizing radiation
[43]. These enzymes are in charge of the hydroperoxide (H
2O
2) reduction, a type of ROS produced in the cell
[44][45], thus decreasing the negative effects of ROS and contributing to the anti-metastatic function
[41][46][47][48]. They are also involved in roles such as DNA-repairing and cytokine control, thus, supplements with Se have been used in chemotherapy since it was reported that it increased the selenoprotein expression in plasma
[49]. Similarly, other selenoproteins that participate in redox processes during tumor progression are the thioredoxin reductases (TXNRDs) with three subtypes placed in the cytosol and nucleus, mitochondria, and sperm
[50]. This selenoprotein subfamily is up-regulated in several cancers such as lung cancer
[51][52], breast cancer
[53][54], and astrocytomas
[55].
2.3. GABA Receptor
γ-Aminobutyric acid (
GABA) is the principal inhibitor in the central nervous system and it is present in the peripheral nervous system as well
[56][57]. The expression of
GABA and
GABA receptors (
GABARAP) is mainly found in brain structures; however, its expression can be detected in other organs like the pancreas, kidney, intestine, prostate, testis, ovaries, and liver where it can trigger hormone and neuronal activity
[56]. Ionizing radiation augments the
GABA receptors mRNA in C17.2 mouse neural stem-like cell lines and in mouse primary neural stem cells, presumably by altering the neuronal function
[58]. Nevertheless, other studies have shown contrary effects that may be because of the differences in doses and time of exposure
[58][59][60]. It was reported that the
GABA signaling pathway was altered in some cancers such as pancreatic, gastric, and breast cancers, an increment in the expression of
GABA and
GABA receptors was found
[61][62][63]. It was observed that activation of these receptors could decrease cell proliferation and migration
[64][65][66]. Thus, the GABARAP system was suggested to play a role in tumorigenesis as a cell migration and proliferation inhibitor
[67].
2.4. Interleukins
Interleukins (IL) are proteins secreted primarily by CD3+ and CD4+. They belong to the cytokine superfamily with about 38 different types of IL and are responsible for the interactions between cells
[68]. In tumors, cytokines collaborate with different elements such as cancer stem cells, microRNA, epithelial-mesenchymal transition (EMT) markers, and transcription factors, thus these biomolecules are involved in different processes, principally in systemic inflammation and immune system modulation, involving cell migration, proliferation, maturation, and adhesion necessary for the inflammatory response
[69][70].
2.5. Epsins 3
Epsins are adaptor proteins, part of a family of ubiquitin-binding endocytic proteins
[71]. In mammals, three genes encode for each isoform;
epsin1,
epsin2, and
epsin3 [72][73][74]. These proteins have a well conserved NH
2-terminal homology domain (ENTH), important in ubiquitination and the ENTH portion comprises about 150 amino acids and it is essential for binding inositol phospholipids and proteins
[75]. Thus, involved in signaling pathways like Notch, Rho GTPase, and VEGFRs
[76][77][78][79].
Epsin type 3 is predominately expressed in the stomach and the epithelia while types 1 and 2 are expressed in different cell types, with no specific location and repetitious functions
[72][73][74]. Besides, it has been shown that these proteins are up-regulated in different cancers
[80][81]. In breast cancer,
Epsin proteins induce NF-kB, which is fundamental in developing the disease, high levels of
epsins are associated with low relapse-free survival rates, especially in ER-negative breast cancer types
[71].
Epsin3 acts as an oncogene in ER-positive breast and other cancers such as non-small cell lung cancer
[82]. Similarly, the
epsin3 protein has been identified in glioblastoma cell lines and samples of patients with glioma, and its overexpression has been shown to induce cell migration and invasion through transcription factors such as Slug, Twist, and ZEB1 promoting EMT in these glioma-type cells
[82]. Studies in the breast cancer model indicated that A3, characterized by cells transformed by radiation only, had higher
epsin gene expression than C.
2.6. Stefin A (Cystatin A)
To understand the role of
stefin genes (
cystatin A, CSTA), it is important to know that cathepsins are lysosomal proteases and they can be classified into serine, cysteine, and aspartyl cathepsins
[83][84], with 11 well-identified types: B, H, L, S, C, K, O, F, V, W, and X/Z
[85]. These proteases are essential in protein degradation processes, associated with phagocytosis, endocytosis, and autophagy
[86], in addition to apoptosis, immune response, development, differentiation, and pro-tumorigenic functions
[83][85][87]. For instance, cathepsin S type is involved in tumor progression
[88], angiogenesis, tumor growth
[89][90], similarly, cathepsin L is involved in neovascularization
[91], migration, and invasion processes as well
[92][93]. Alteration and changes in expression of cathepsins are associated with pathological circumstances, for example, they are secreted into the extracellular medium in cancer
[94][95][96].
2.7. Metallothioneins
Metallothioneins (MTs) are small (approximately 6–7 kDa) cytosolic proteins with a high content of cysteine groups (30%)
[97][98]. There are four main MT isoforms in humans—MT1, MT2, MT3, and MT4—encoded by a gene located at the 16q13 locus
[99]. There is evidence that connects MTs with tumor formation, progression, and drug resistance
[99]. Their principal role is in homeostasis and the detoxification of heavy metals, oxidative stress, and DNA damage processes
[99][100]. They bind to heavy metals (with great affinity) via the thiol binding part of the cysteine-enriched portion
[99]. When MTs bind to metals such as zinc and copper, they can regulate different important processes such as cell growth, proliferation, differentiation, metastasis, and protection against oxidative radicals produced by drugs and radiation
[99][101][102][103]. However, they also can bind to other metals like cadmium, mercury, and platinum, among others, to protect cells from these heavy metals
[104]. The expression of MTs depends on the type of tumor suggesting a specific role in carcinogenesis
[97][105][106][107]. MTs have been reported to be up-regulated in breast, ovarian urinary bladder, and nasopharyngeal cancer, and melanoma
[108][109][110][111][112]. A positive correlation has been established between MTs and Ki-67, a marker of cellular proliferation in breast cancer
[108][113][114]. Specific MTs such as MT1F and MT2A have been found in greater numbers in cancer in stage 3 than in stage 1 and 2 when histological samples of breast cancer were analyzed
[114][115]. In addition, zinc was demonstrated to increase the expression of the vascular epithelial growth factor (VEGF) in three breast cell lines
[116].
It can be summarized that in the breast cancer model the cell line transformed only by radiation independently of estrogen was characterized by greater gene expression of ATM, selenoproteins, GABA receptor, interleukins, specifically IL-7, epsin, stefin and metallothioneins than in other cell lines. Understanding the effect of radiotherapy in different types of cells will help us improve the clinical outcome of radiotherapy. Thus, gene signatures have been demonstrated to be specific to tumor types, hence cell-dependency must be considered in future treatment planning. Molecular and clinical features affect the results of radiotherapy. Thus, using gene technology and molecular information it is possible to improve therapy and reduce the side effects of therapeutic radiation use.