COVID-19 is an infectious disease caused by the SARS-CoV-2 virus. The clinical presentations of the SARS-CoV-2 infection are widely variable and treatment strategies for COVID-19 are dependent on the infection phase. Timing the right treatment for the right phase of this disease is paramount, with correlations detected between the phase of the infection and the type of drug used to treat. The immune system activation following COVID-19 infection can further develop to a fulminant cytokine storm which can progress to acute respiratory distress syndrome. The inflammatory phase, or the hyperinflammation phase, is a later stage when patients develop acute respiratory distress syndrome (ARDS), sepsis, and kidney and other organ failure. In this stage, the virus is probably not necessary and all the damage is due to the immune system’s cytokine storm. Immunosuppressive or immunomodulatory agent administration is the major strategy in treating COVID-19 patients at this stage. On the other hand, immunodeficient patients who are treated with immunomodulator agents have attenuated immune systems that do not produce enough cytokines. Current data do not show an increased risk of severe COVID-19 in patients taking biologic therapies or targeted disease-modifying antirheumatic drugs.
1. Introduction
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. The presentation of the SARS-CoV-2 infection ranges in clinical severity, from asymptomatic to a mild upper respiratory tract illness, to a diffuse viral pneumonia causing acute respiratory failure, with sequelae including acute lung injury, multiorgan dysfunction syndrome, and death [
1,
2,
3]. Most people infected with the virus will be asymptomatic or experience mild to moderate respiratory illnesses and recover without requiring special treatment. However, some will become seriously ill and require medical attention [
4,
5]. Older people and those with underlying medical conditions, such as cardiovascular diseases, diabetes, chronic respiratory diseases, or cancer, are more likely to develop a serious illness [
3,
4,
5].
The viral infection is divided into three stages [
2,
3]. The first stage is the early infection or viral response phase, during which symptoms of upper respiratory tract infection dominate. The second stage is the pulmonary phase, when patients develop viral pneumonia, probably bilateral, with all its associated symptoms [
2]. This pulmonary phase is divided into two distinct parts [
1,
2,
3]. Stage IIA is pneumonia without hypoxia and stage IIB is pneumonia with hypoxia. Patients at stage IIB will likely require hospitalization and oxygen supplementation [
1,
2,
3]. The third stage is the inflammatory phase or the hyperinflammation phase, when patients develop acute respiratory distress syndrome, sepsis, and kidney and other organ failures. In the third stage, otherwise known as the cytokine storm, the virus is probably not directly involved, and all the damage is caused by an overreactive immune system [
1,
2,
3]. There is a direct correlation between the cytokine storm and morbidity and mortality [
1,
2,
3].
Treatment strategies in COVID-19 depend on the infection phase [
5,
6,
7,
8,
9,
10,
11,
12]. In the early viral phases, the treatments of choice are antiviral agents such as Remdesivir, which is an RNA-dependent polymerase inhibitor [
6], and convalescent plasma, i.e., plasma from patients that have been cured from COVID-19 infection [
9]. Other treatments include hydroxychloroquine, a well-known drug used for several decades for the treatment of rheumatoid arthritis, systemic lupus erythematosus, and malaria prophylaxis [
10,
11], and lopinavir-ritonavir [
8], which is an HIV protease inhibitor and is indicated in combination with other antiretroviral products for the treatment of human immunodeficiency virus [
8]. However, recently, the NIH Panel for COVID-19 Treatment Guidelines recommends against the use of lopinavir/ritonavir or other HIV protease inhibitors in COVID-19 infections [
8]. This is due to unfavorable pharmacodynamic data and mainly due to the lack of clinical benefit in patients with COVID-19 [
8]. A new confirmed antiviral agent is the Regen-COV-2 antibody cocktail, which consists of two monoclonal antibodies, casirivimab and imdevimab. This cocktail has successfully helped patient immune systems eradicate the virus [
12].
The main risk factor in this SARS-CoV-2 infection is the development of a fulminant cytokine storm [
4]. This storm happens when the immune system’s reaction due to the SARS-CoV-2 virus becomes hyperactive, resulting in an excessive inflammatory reaction with the release of large amounts of pro-inflammatory cytokines. The cytokine storm in COVID-19 can mimic hemophagocytic lymphohistiocytosis or macrophage activation syndrome, which can progress to acute respiratory distress syndrome (ARDS) and multiorgan failure [
5], with a serious risk of death to the patient [
5].
Vaccination of the population against the coronavirus disease has reduced both the mortality and severity of the disease [
5,
13]. This development cycle of the vaccine against SARS-CoV-2 was the turning point. The current main challenge is the emergence of new variants that have developed in non-vaccinated countries that can infect vaccinated people [
5]. This is due to a restricted immune memory [
5,
13], which is about six months in twice-dosed vaccinated people [
5,
13]. Beyond six months, the data show a similar increase in the rate of infections [
5]. The rate of severe cases also increases as a function of time from vaccination. Serological studies show a correlated time-dependent reduction in neutralization titers [
5,
13].
2. Cytokine Storm in COVID-19: The Correlation between the Cytokine Storm, Morbidity, and Mortality
Globally, humans have suffered from many viral pandemics in the last century [
21], with recent ones being severe acute respiratory syndrome (SARS) [
22], the influenza virus pandemic [
23], and the H1N1 swine influenza [
23]. These viral infections pose risks to our health in that some of them are zoonotic infections with the threat of human-to-human transmission and excessively high mortality rates [
24]. At the end of 2019, another strain of SARS-CoV was identified, SARS-CoV-2, which has caused the COVID-19 pandemic.
The main effects of SARS-CoV-2 on the immune system are in the distribution and apoptosis of lymphocytes. There is a decrease in the total number of lymphocytes, cytotoxic and helper T cells, B, and NK cells, and almost all their subsets, especially in patients with a severe course of COVID-19 [
4]. Development of fulminant cytokine storm due to the SARS-CoV-2 virus infection results in an excessive inflammatory reaction with the release of large amounts of pro-inflammatory cytokines (
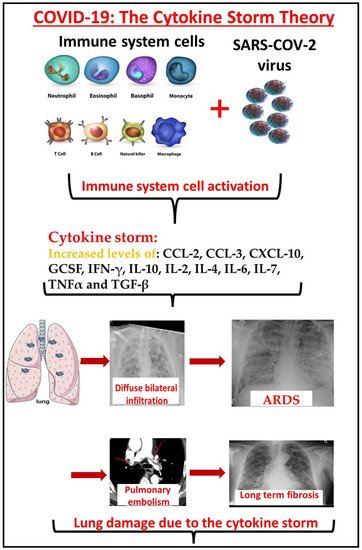
Figure 1). This condition mimics a dangerous situation called hemophagocytic lymphohistiocytosis, or macrophage activation syndrome, with a serious risk of death [
5]. Cytokine storms are strongly correlated with severe disease and death [
12,
19,
24].
Figure 1. The effect of the cytokine storm on COVID-19 patients. The SARS-CoV-2 virus infection activates the immune system, which releases large concentrations of cytokines such as CCL-2, CCL-3, CXCL-10, GCSF, IFN-γ, IL-10, IL-2, IL-4, IL-6, IL-7, TNFα, and TGF-β. These cytokines mediate lung capillary primiparity and penetration. Diffuse bilateral ground glass infiltrate develops and the patient becomes hypoxemic. Endothelial injury mediated coagulation disorders, and pulmonary embolism could develop in these patients. The fibrotic damage to the lungs is probably long term.
The inflammatory third phase of the SARS-CoV-2 infection is the dangerous one. It is not related to the viral load but to an uncontrolled immune activation [
24,
25,
26,
27,
28,
29,
30]. Elevation in inflammatory mediators, including cytokines and chemokines such as interleukin (IL)-2, IL-7, IL-10, tumor necrosis factor (TNF), granulocyte colony-stimulating factor (G-CSF), monocyte chemoattractant protein-1, and other inflammatory cytokines, such as C-reactive protein, ferritin, and D-dimers, significantly correlate with disease severity [
25,
26,
27,
28,
29,
30,
31,
32,
33]. Some of these cytokines predict mortality in COVID-19 patients [
9,
19,
20,
34,
35]. These cytokines mediate lung capillary permeability and penetration. This mediated, diffuse, bilateral ground glass infiltrate can develop into hypoxemia. Endothelial injury mediated coagulation disorders and fibrotic damage to the lungs (
Figure 1).
Serum pro-inflammatory cytokine levels were measured upon admission and again upon discharge in severely ill patients [
19]. CXCL-10, GCSF, IL-2, and IL-6 serum concentrations were significantly reduced upon discharge [
19]. A comparison of these cytokines between severely ill patients who died during their hospitalization period and mildly ill patients who were discharged two days later show significant elevation in the non-surviving group [
19,
36].
3. The Cytokine Storm as a Target Choice in the Treatment of COVID-19
The cytokine storm plays an essential role in the pathogenesis and clinical outcome of virus infections [
24]. Blocking the cytokine storm provides greater protection than does antiviral therapy in the influenza virus [
24].
The cytokine storm in fatal COVID-19 is represented by several pathological features, such as ARDS, coagulation, and multiorgan dysfunctions [
12,
19,
24]. Patients with serious COVID-19 infections may suffer from a release of cytokines which can damage the lung tissues [
12,
19,
24]. A severe COVID-19 cytokine storm is characterized by the release of proinflammatory mediators [
12,
19,
24,
67], and the amplified pulmonary inflammatory response results in enlarged alveolar–capillary gas exchange, making oxygenation difficult [
67].
Elevated IL-6 and IL-1 concentrations correlate with intrapulmonary macrophage activation and pulmonary vascular disease [
67]. These cytokines significantly contribute to fever, lymphopenia, coagulation, lung injury, and multiorgan failure [
67,
68]. IL-1β is a mediator of lung inflammation, fever, and fibrosis. Suppression of IL-1 family members and IL-6 has been shown to have a therapeutic effect in many inflammatory diseases, including viral infections such as SARS-CoV-2 [
67,
68,
69]. Therefore, many drugs which target cytokines in COVID-19 were tested for their effect on mortality and morbidity [
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80].
IL-6 levels are highly correlated with the lethal complications of COVID-19, and are associated with poor prognosis and progression, as well as disease augmentation [
35,
69,
70,
71,
72,
73,
74,
75,
76]. Agents which mediate IL-6 inhibition may improve the patients’ conditions. Tocilizumab is a recombinant, humanized, monoclonal anti-interleukin IL-6 antibody that targets the human IL-6 receptor (IL-6R). However, results using tocilizumab are mixed [
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80]. While some studies show that tocilizumab is not efficacious in improving hospitalized patients infected with severe acute respiratory syndrome coronavirus [
75], other studies show that decreasing IL-6 levels by tocilizumab correlates with a decrease of the risk of mortality caused by COVID-19 [
69,
76]. Furthermore, many studies confirm that the use of tocilizumab decreases the need for mechanical ventilation [
77,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89].
Anti-IL-1 molecules were also tested for their efficacy on reducing morbidity and mortality in COVID-19 patients [
90,
91]. Anakinra is the main treatment in this group. Anakinra treatment, compared to the standard of care, shows a decrease in twenty-eight-day mortality and in hospital stay [
91].
Anti-TNF agents are commonly used for the treatment of rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. While serum TNF-α levels are moderately elevated in patients with SARS [
92,
93], much higher serum levels are observed in COVID-19 patients, positively correlating with disease severity [
92,
93]. Although anti-TNF treatment was suggested as a potential treatment for COVID-19, there is insufficient data [
92]. Some studies have demonstrated that there is a correlation between anti-TNF-α therapy with an increase in the risk of intracellular infection of SARS-CoV-2 infection via induction of the Notch-1 signaling pathway [
93].
The efficacy of corticosteroids (CS) on inflammatory organ injury in viral pneumonia remains controversial [
72,
74,
94]. Immunosuppressive glucocorticoids when administered to severely ill patients who need oxygen show the greatest benefit in preventing deterioration of these patients [
36,
37]. Steroids are not recommended to non-hypoxic patients [
35,
36,
37]. In the absence of an oxygen requirement, patients on glucocorticoids may fare worse than those who receive standard care [
35,
36,
37]. Since there is no positive evidence coming from randomized clinical trials, the WHO guidelines do not recommend routinely using systemic CS treatment for patients with COVID-19 [
94]. Thus, the selection of patients and timing of the administration of glucocorticoids is critical for survival benefits [
35]. Timing is paramount in this epidemic. Suppressing the host response too early during rapid viral replication is probably deleterious, whereas waiting for the requirement for respiratory support may be the appropriate time to intervene with these medications [
35].
In clinical practice, intravenous immunoglobulin (IVIG) is used in patients with immune deficiencies for treating infectious diseases, as well as for treatment-resistant patients with autoimmune diseases as an immunomodulatory agent [
95,
96]. IVIG polyclonal immunoglobulin G (IgG) was also studied for its effect on morbidity and mortality in COVID-19 patients [
95]. Previous favorable experiences from patients with SARS and MERS suggest the efficacy of a high dose of IVIG (0.3–0.5 g/kg) in patients with a serious COVID-19 infection in the early phase of the disease [
95,
96]. IVIG has demonstrated clinical efficacy in critically ill patients with COVID-19 [
95]. There may be a relationship between the efficacy of IVIG and the COVID-19 disease severity [
95,
96].
Convalescent plasma is plasma from patients that were cured of a COVID-19 infection. Convalescent plasma may offer various beneficial actions in COVID-19 disease. In non-critical hospitalized patients, convalescent plasma therapy reduces the morbidity and mortality in moderately ill COVID-19 patients and shortens the hospitalization length [
19]. The proposed mechanism of how convalescent plasma helps COVID-19 patients in the first stage of infection is not completely known [
19]. Some studies show that antibodies from convalescent plasma can suppress viremia [
59]. Administering convalescent plasma at the early stage of the disease was found to be more effective [
19,
59].
Mast cells may be another therapeutic target point in SARS-CoV-2 infection. This virus could activate mast cells. These cells mediate allergic and pulmonary diseases by secreting cytokines and material such as histamine, leukotrienes, and proteases [
97]. Inhibition of mast cells could attenuate the effect of these cells on the organs, especially the lungs. The flavone luteolin could bind to the surface spike protein of SARS-CoV-2 and inhibit entry of the virus into host cells. This material also inhibits serine proteases, which is required for viral infectivity. Luteolin also inhibits mast cells and has anti-inflammatory properties, inhibiting secretion of the pro-inflammatory cytokines from human mast cells [
97]. This drug could be effective in targeting the cytokine storm mediated by SARS-CoV-2 infection.
In summary, the cytokine storm is the most dangerous factor in this disease. This stage causes permanent or long-lasting damage to the body most of the time. Some of these damages are still unknown and unclear. Targeting some of the players in the cytokine storm can possibly attenuate the storm and prove to be beneficial. More evidence is currently being accumulated about the effect of this “storm” and on possible treatment modalities.
This entry is adapted from the peer-reviewed paper 10.3390/covid2050040