Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Contributors: Michael Boettcher, Alexander Joechner, Ziduo Li, Sile Fiona Yang, Patrick Schlegel
Chimeric antigen receptor (CAR) T cells are artificially generated transgenic cells that express a hybrid in silico designed de novo dimeric immune receptor. The basic architecture of CAR receptors is an extracellular antigen recognition domain, a spacer domain, a transmembrane domain, and an intracellular signaling domain. Each domain of a CAR receptor has been intensively studied and variations have been designed and established successfully.
- chimeric antigen receptor
- CAR T cell therapy
- evolution of CAR T cells
1. Introduction
CAR T cell therapy has revolutionized immunotherapy in the last decade with the successful establishment of chimeric antigen receptor (CAR)-expressing cellular therapies as an alternative treatment in relapsed and refractory (r/r) homogeneously CD19-positive leukemias and lymphomas [1][2][3]. There are fundamental reasons why CAR T cell therapy has been approved by the Food and Drug administration (FDA) in the USA and the European Medicines Agency (EMA) for pediatric and young adult patients, as well as adult patients whose clinical data usually pave the way for translation of novel therapies into the clinic for children. Commonly, novel therapies are developed for the larger adult patient cohort, and then adapted for pediatric use, due to regulatory and commercial reasons [4][5]. Both strategic and biological factors have supported the development of CAR T cell therapy in children. The higher clinical relevance of CD19-positive malignancies in children compared to adults is one of the pivotal factors. B-cell acute lymphoblastic leukemia (B-ALL) is the most common pediatric malignancy, with a prevalence of up to 25% of cancers in all childhood cancers [6]. In contrast, the prevalence of all cancers in adults is below 0.5%, and B-cell non-Hodgkin’s lymphoma (NHL) represents approximately 3.6% of adult cancers [7][8]. Despite the unprecedented success story of ALL treatment in childhood, with 5 year overall survival rates exceeding 90% in contemporary treatment optimization studies [9], prognosis for r/r patients and patients with high-risk predispositions is still dismal [10]. Therefore, there is an urgent need for improved and more specific therapies in r/r ALL to reduce the adverse event profile and prolong survival. Furthermore, the susceptibility of B-ALL to CAR T cell therapy is significantly higher [2] than that of chronic lymphoblastic leukemia (CLL) [11] and a broad variety of B-lineage-derived lymphomas [12].
In general, pediatric ALL is an unmatched success story in cancer treatment, with high overall survival (OS) rates throughout the Western world, drastically increasing from no chance of survival in the 1950s, ~10% OS in the 1960s, ~40% OS in the 1970s, ~65% in the 1980s, to survival rates above 90% today [9]. The main reason for the excellent survival rates is the sophisticated chemotherapy protocols that have been initiated and optimized over the last seven decades [13]. Moreover, major advances have been achieved with the development and improvement of allogeneic hematopoietic stem cell transplantation (allo-HSCT) [14] and immunotherapy with the bispecific T cell engager therapy (BiTE) blinatumomab (CD3XCD19) [15][16], which is currently trialed in patients with precursor B-ALL as an alternative to conventional intensive and toxic chemotherapies, and in patients who are at high risk of relapse post chemotherapy in the clinical trial AIEOP-BFM ALL 2017 (NCT03643276).
CD19-CAR T cell therapy has been a medical breakthrough in the treatment of pediatric ALL, demonstrated by its outstanding clinical success, which exceeds previous therapies including allo-HSCT and blinatumomab treatment in r/r patients considered to be incurable with a shortened life expectancy [2][17]. CD19-targeted CAR-expressing T cells (CD19-CAR-T) were able to cure pediatric patients with a single-agent infusion trialed as the last resort after blinatumomab therapy [2]. Subsequent exploration of CD19-CAR-T cell treatment also demonstrated success in r/r ALL patients post allo-HSCT after infusion of true-allogeneic CD19-CAR T cells (donor-derived) [18] and pseudo-allogeneic (posttransplant recipient-derived) CD19-CAR T cells [19]. In the landmark clinical trials NCT01626495 and NCT01029366, autologous CD19-CAR-T treatment resulted in a high response rate (90% complete remission induction) and a 50% long-term event-free survival, despite recruitment of a limited number (N = 25) of patients [2]. These unprecedented clinical data in CAR T cell trials have led to the FDA approval of the first CD19-CAR-T cell therapy in children and young adults with B-ALL in 2017.
2. Molecular Architecture of CAR Receptors
CAR T cells are artificially generated transgenic cells that express a hybrid in silico designed de novo dimeric immune receptor. The basic architecture of CAR receptors is an extracellular antigen recognition domain, a spacer domain, a transmembrane domain, and an intracellular signaling domain [20]. Each domain of a CAR receptor has been intensively studied and variations have been designed and established successfully. It is noteworthy that critical steps in the development of CAR receptors were necessary to make CAR T cells potent therapeutics being capable of curing patients [21][22].
The main function and idea of CAR receptors are obviously to enable immune effector cells such as T cells and NK cells to be specifically redirected to cancer cells overexpressing the target antigen in a major histocompatibility complex (MHC)-independent manner [23][24]. scFv-based CAR receptors may also be constructed to target peptides presented by the MHC, for instance HLA-A2/NY-ESO-1 [25]. In Figure 1, the CAR architecture is illustrated and indicates established domain-variations.
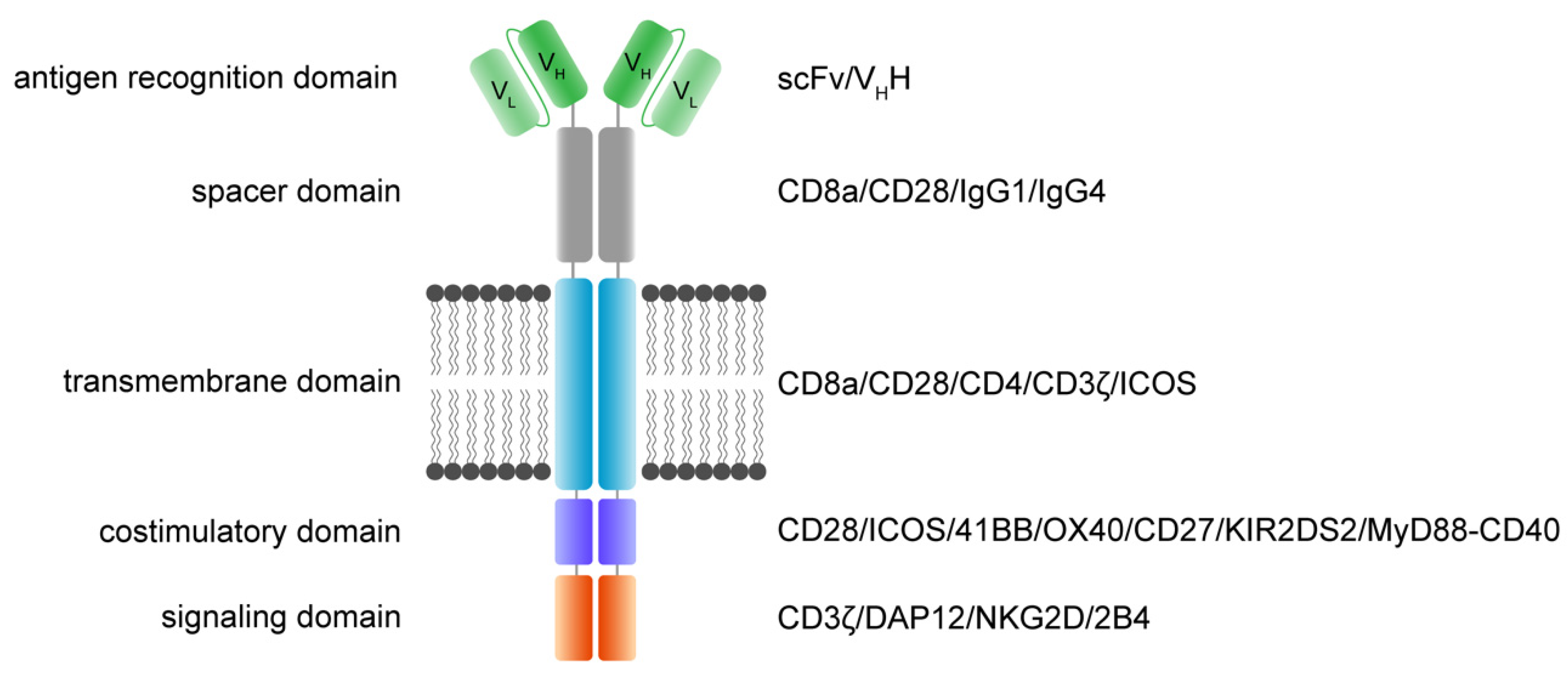
Figure 1. Functional modules of CAR receptors.
A schematic illustration of a second-generation CAR receptor. CAR receptors are comprised of several modules indicated in different colors—the antigen recognition domain, which usually consists of an antibody-derived scFv or VHH, the spacer domain of variable length, configuration, and flexibility, connecting the antigen recognition domain to the transmembrane domain. The transmembrane domain robustly anchors the CAR in the phospholipid bilayer cell membrane and is linked to the intracellular parts of the artificial immune receptor. Thus, another important role of the transmembrane domain is to facilitate the mechanic signal transduction into the cell. The intracellular costimulatory domains and signaling domain transform the activation signal via a signaling cascade into the cell to activate downstream signaling that results in various effector functions such as cytolysis, cytokine secretion and proliferation. scFv: single-chain variable fragment; VHH: heavy chain variable fragment of a single-domain antibody; VL: variable fragment of the light chain; VH: variable fragment of the heavy chain.
A CAR is a modular structure typically consisting of an extracellular antigen-binding domain linked by a spacer region to a transmembrane domain, attached to one or more intracellular activation domains. In general, every subunit of a CAR can significantly change the properties and function of the CAR receptor. CAR design has evolved over the last three decades, with the goal to improve CAR T cell efficacy, persistence, and safety.
The extracellular recognition domain in most CAR receptors is derived from the variable segments of the antibody light and heavy chains. They are constructed in line with peptide linkers [20][23] to assemble in a single-chain variable fragment (scFv) format. In general, scFvs are less stable in their configuration compared to the Fab region of antibodies [26]. Most antibodies in the past were generated by immunization of mice [27]. Today, fully human antibodies can be generated [28]. Single-domain VH binders (sdFv) based on human libraries or camelid binders or alternative formats can also be used as recognition domains [29]. The advantage of camelid sdFv is the reduced genetic load (half the size), reduced immunogenicity and the reduced tendency for aggregation while retaining the same specificity and affinity [30]. For hidden epitopes, the sdFv may be advantageous for the initial interaction of the targeted epitope compared to scFv based targeting due to less steric hinderance, higher solubility and the stability. Further, ligand-based CAR recognition domains have been introduced to target BCMA via trimeric APRIL [31], and the small chlorotoxin, a naturally derived 36-amino-acid-long peptide found in the venom of the death stalker scorpion leiurus quinquestriatus, which selectively binds to primary brain cancers is used for the treatment of glioblastoma (GBM) [32]. The basic requirement of recognition domains is the specific and rapid binding to the targeted antigen with the recognition domain to facilitate the CAR engagement.
The structural domains including the spacer (also called hinge) and transmembrane domains stabilize the receptor and allow the functional presentation of the recognition domain. They shape the extracellular configuration of the receptor and connect the extracellular domains to the intracellular modules of the receptor to facilitate an efficient mechanistic signal transduction to the intracellular signaling domains. Various protein subunits derived from CD8a, CD28, and IgG hinge regions also in combination with IgG CH2 and CH3 domains and others have been utilized as spacer domains, which have shown distinct properties. The most frequently used transmembrane domains are derived from the CD8a and CD28 [33].
The intracellular signaling domains usually contain one or more costimulatory domains and a signaling domain. Costimulatory domains are mainly derived from two families, namely the immunoglobulin superfamily, which is represented by CD28 and ICOS, and the tumor necrosis factor receptor superfamily (TNFR) represented by 4-1BB, OX40 and CD27. Signaling domains are mainly derived from the CD3ζ chain, while alternative signaling domains such as DAP12 have been used [34][35][36].
This entry is adapted from the peer-reviewed paper 10.3390/jcm11082158
References
- Singh, A.K.; McGuirk, J.P. CAR T cells: Continuation in a revolution of immunotherapy. Lancet Oncol. 2020, 21, e168–e178.
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517.
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544.
- Spadoni, C. Pediatric Drug Development: Challenges and Opportunities. Curr. Res. Clin. Exp. 2018, 90, 119–122.
- Joseph, P.D.; Craig, J.C.; Caldwell, P.H.Y. Clinical trials in children. Br. J. Clin. Pharmacol. 2015, 79, 357–369.
- Kakaje, A.; Alhalabi, M.; Ghareeb, A.; Karam, B.; Mansour, B.; Zahra, B.; Hamdan, O. Rates and trends of childhood acute lymphoblastic leukaemia: An epidemiology study. Sci. Rep. 2020, 10, 6756.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577.
- Inaba, H.; Mullighan, C.G. Pediatric acute lymphoblastic leukemia. Haematologica 2020, 105, 2524–2539.
- Reismüller, B.; Peters, C.; Dworzak, M.N.; Pötschger, U.; Urban, C.; Meister, B.; Schmitt, K.; Dieckmann, K.; Gadner, H.; Attarbaschi, A.; et al. Outcome of children and adolescents with a second or third relapse of acute lymphoblastic leukemia (ALL): A population-based analysis of the Austrian ALL-BFM (Berlin-Frankfurt-Münster) study group. J. Pediatr. Hematol. Oncol. 2013, 35, e200–e204.
- Lemal, R.; Tournilhac, O. State-of-the-art for CAR T-cell therapy for chronic lymphocytic leukemia in 2019. J. ImmunoTher. Cancer 2019, 7, 202.
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 380, 45–56.
- Pui, C.-H.; Evans, W.E. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin. Hematol. 2013, 50, 185–196.
- Brissot, E.; Rialland, F.; Cahu, X.; Strullu, M.; Corradini, N.; Thomas, C.; Blin, N.; Thebaud, E.; Chevallier, P.; Moreau, P.; et al. Improvement of overall survival after allogeneic hematopoietic stem cell transplantation for children and adolescents: A three-decade experience of a single institution. Bone Marrow Transplant. 2016, 51, 267–272.
- Handgretinger, R.; Zugmaier, G.; Henze, G.; Kreyenberg, H.; Lang, P.; Von Stackelberg, A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia 2011, 25, 181–184.
- Patrick, S.; Peter, L.; Gerhard, Z.; Martin, E.; Hermann, K.; Kai-Erik, W.; Judith, F.; Matthias, P.; Heiko-Manuel, T.; Christina, K.; et al. Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica 2014, 99, 1212–1219.
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528.
- Kebriaei, P.; Singh, H.; Huls, M.H.; Figliola, M.J.; Bassett, R.; Olivares, S.; Jena, B.; Dawson, M.J.; Kumaresan, P.R.; Su, S.; et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J. Clin. Investig. 2016, 126, 3363–3376.
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 2016, 126, 2123–2138.
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028.
- Milone, M.C.; Fish, J.D.; Carpenito, C.; Carroll, R.G.; Binder, G.K.; Teachey, D.; Samanta, M.; Lakhal, M.; Gloss, B.; Danet-Desnoyers, G.; et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol. Ther. 2009, 17, 1453–1464.
- Gong, M.C.; Latouche, J.-B.; Krause, A.; Heston, W.D.; Bander, N.H.; Sadelain, M. Cancer Patient T Cells Genetically Targeted to Prostate-Specific Membrane Antigen Specifically Lyse Prostate Cancer Cells and Release Cytokines in Response to Prostate-Specific Membrane Antigen. Neoplasia 1999, 1, 123–127.
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724.
- Goverman, J.; Gomez, S.M.; Segesman, K.D.; Hunkapiller, T.; Laug, W.E.; Hood, L. Chimeric immunoglobulin-T cell receptor proteins form functional receptors: Implications for T cell receptor complex formation and activation. Cell 1990, 60, 929–939.
- Ochi, T.; Maruta, M.; Tanimoto, K.; Kondo, F.; Yamamoto, T.; Kurata, M.; Fujiwara, H.; Masumoto, J.; Takenaka, K.; Yasukawa, M. A single-chain antibody generation system yielding CAR-T cells with superior antitumor function. Commun. Biol. 2021, 4, 273.
- Kang, T.H.; Seong, B.L. Solubility, Stability, and Avidity of Recombinant Antibody Fragments Expressed in Microorganisms. Front. Microbiol. 2020, 11, 1927.
- Asensio, M.A.; Lim, Y.W.; Wayham, N.; Stadtmiller, K.; Edgar, R.C.; Leong, J.; Leong, R.; Mizrahi, R.A.; Adams, M.S.; Simons, J.F.; et al. Antibody repertoire analysis of mouse immunization protocols using microfluidics and molecular genomics. mAbs 2019, 11, 870–883.
- Lonberg, N. Fully human antibodies from transgenic mouse and phage display platforms. Curr. Opin. Immunol. 2008, 20, 450–459.
- Zajc, C.U.; Salzer, B.; Taft, J.M.; Reddy, S.T.; Lehner, M.; Traxlmayr, M.W. Driving CARs with alternative navigation tools—The potential of engineered binding scaffolds. FEBS J. 2021, 288, 2103–2118.
- Asaadi, Y.; Jouneghani, F.F.; Janani, S.; Rahbarizadeh, F. A comprehensive comparison between camelid nanobodies and single chain variable fragments. Biomark. Res. 2021, 9, 87.
- Schmidts, A.; Ormhøj, M.; Choi, B.D.; Taylor, A.O.; Bouffard, A.A.; Scarfò, I.; Larson, R.C.; Frigault, M.J.; Gallagher, K.; Castano, A.P.; et al. Rational design of a trimeric APRIL-based CAR-binding domain enables efficient targeting of multiple myeloma. Blood Adv. 2019, 3, 3248–3260.
- Wang, D.; Starr, R.; Chang, W.-C.; Aguilar, B.; Alizadeh, D.; Wright, S.L.; Yang, X.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci. Transl. Med. 2020, 12, eaaw2672.
- Jayaraman, J.; Mellody, M.P.; Hou, A.J.; Desai, R.P.; Fung, A.W.; Pham, A.H.T.; Chen, Y.Y.; Zhao, W. CAR-T design: Elements and their synergistic function. EBioMedicine 2020, 58, 102931.
- Zhang, H.; Zhao, P.; Huang, H. Engineering better chimeric antigen receptor T cells. Exp. Hematol. Oncol. 2020, 9, 34.
- Ng, Y.-Y.; Tay, J.C.; Li, Z.; Wang, J.; Zhu, J.; Wang, S. T Cells Expressing NKG2D CAR with a DAP12 Signaling Domain Stimulate Lower Cytokine Production While Effective in Tumor Eradication. Mol. Ther. 2020, 29, 75–85.
- Guedan, S.; Calderon, H.; Posey, A.D., Jr.; Maus, M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther. Methods Clin. Dev. 2019, 12, 145–156.
This entry is offline, you can click here to edit this entry!
