Today, as a result of the advancement of technology and increasing environmental problems, the need for clean energy has considerably increased. In this regard, hydrogen, which is a clean and sustainable energy carrier with high energy density, is among the well-regarded and effective means to deliver and store energy, and can also be used for environmental remediation purposes. Renewable hydrogen energy carriers can successfully substitute fossil fuels and decrease carbon dioxide (CO2) emissions and reduce the rate of global warming. Hydrogen generation from sustainable solar energy and water sources is an environmentally friendly resolution for growing global energy demands. Among various solar hydrogen production routes, semiconductor-based photocatalysis seems a promising scheme that is mainly performed using two kinds of homogeneous and heterogeneous methods, of which the latter is more advantageous. During semiconductor-based heterogeneous photocatalysis, a solid material is stimulated by exposure to light and generates an electron–hole pair that subsequently takes part in redox reactions leading to hydrogen production.
1. Introduction
1.1. Hydrogen as a Clean Energy Carrier
Today, as a result of the increasing population and the increasing need for clean resources due to environmental limitations, the search for sustainable and clean origins is becoming an increasingly crucial issue. Unfortunately, most of the energy requirement is mainly fulfilled by fossil fuels with finite reserves, and their consumption leads to the generation of greenhouse gases, leading to climate changes along with other significant adverse environmental issues. In this regard, renewables or alternative energy sources, such as solar, wind and biomass, were introduced to reduce greenhouse gases, especially carbon dioxide. Traditional energy production technologies account for an energy supply that is one order of magnitude higher than that from solar photovoltaic. The miniaturization of equipment allows rapid development because of lower investment costs and increased efficiency. Now, in many parts of the world, solar provides the cheapest electricity, and the milestone of attaining a cumulative capacity of more than 1 TW is expected by 2023
[1]. Present integration strategies and those under development will provide extensive penetration of solar photovoltaics, not only in the power grid but also in the entire energy system.
Crucial transformation and a substantial renovation of the energy system in the world are required to decarbonize and attain the Paris Agreement targets. This significant reform is accompanied by an urgent and complex issue that needs system solutions and global joint and coordinated efforts, with hydrogen representing one of these solutions. Hydrogen is one of the key players in achieving a low-carbon energy system. It has the capability to be an influential accelerator for this system, with the ability to address multiple energy challenges, such as facilitating the vast integration of renewable resources and decarbonization of energy production, energy transportation in a zero-carbon energy economy, and electrification of end uses. Hydrogen is one of the most promising sustainable energy carriers that can be substituted for fossil fuels, and has a high gravimetric energy density ranging from 120 to 142 MJ/kg, compared to 44.5 MJ/kg for gasoline
[2]. In addition, hydrogen combustion is clean (CO
2 free) and leads to water production (2H
2 + O
2 ⇌ 2H
2O; ∆E= −286 kJ/mole). However, nearly 96% of hydrogen production is highly interdependent on fossil fuels; hence the need to find renewable resources is crucial. Recently, hydrogen has seen unprecedented interest and development. Soon, it will become a systemic element in the efforts to transition to a climate-neutral society, and, by replacing coal, oil, and gas, it will play the role of a crucial energy vector and the other leg of the energy transition—in addition to renewable electricity—across various parts of the economy.
The growing demand for renewable energy sources in the electricity sector has increased the need for supplementary technologies to balance the grid. However, electricity cannot be stored easily, which presents an opportunity for the application of hydrogen and the development of its technologies. Hydrogen technologies can play an influential role in energy distribution in order to provide conditions for reducing carbon consumption. Delivery has a crucial role in energy use, cost, and emissions, which are closely related to the available options of hydrogen delivery, including transportation via pipelines or road transport. This choice is dependent upon location, local geography, and markets. The combined use of renewable resources is important to the provision of constant power and the achievement of a stabilized electrical grid. Hydrogen can be converted back to electricity when other renewable sources are unavailable. Moreover, the excess can be sold for other purposes. In addition, hydrogen can be used as the primary feedstock of industries using fertilizer, refining, and other chemical-based technologies, and can also be a side product of other industrial processes, making it a strategically important commodity. It can be simultaneously injected into the natural gas grid to maintain a clean gas distribution by reducing emissions and other stranded assets. To stabilize electric grids, controlling the flow of electricity output from renewable energy power generators is crucial. Electricity storage facilities are required to make effective use of renewable energy and avoid output control. A key role in this matter for the immediate future is storage batteries. With the development of renewable energy, the urgency of output controls will also increment; however, hydrogen will also be used in large-scale and long-term power-to-gas systems. Renewable hydrogen can be used in many ways. It is the feedstock for producing methane, clean chemicals, and fertilizers. Hydrogen is considered to be fundamental in helping the value chain of different industries. Hydrogen can be obtained from natural gas reforming and/or pyrolysis, and transported through traditional pipelines mixed with methane. It is an energy carrier for many chemical transformations to be employed in industry such as ammonia. Ammonia (NH3): (1) has a higher hydrogen density (1.5 times higher than liquefied hydrogen) than other carriers, its production needs cheaper infrastructure, and it can be developed through smaller-scale industries; (2) has easily available and existing commercial supply chains; (3) is produced from natural gas and relatively cheaper. Ammonia can be directly used for power generation without a need for the dehydrogenation process and emits no CO2 during combustion. Hydrogen can be used for building heating as a fuel, to leverage hydrogen technologies individually, or a combination of both, which offers high efficiency for heat and power generation. The handling of hydrogen as a fuel is similar to that of natural gas. Hydrogen can be combined with fuel cell technologies that generate electricity and heat to ultimately reduce carbon in several areas such as transportation, power generation, many kinds of industrial processes, and heat use. Natural gas power generation features are essential for the expansion of renewable energy power generation. Hydrogen power generation can be applied the same way as natural gas power generation and may become an outstanding option for reducing carbon in fossil power generation. Hydrogen can also be combined with CO2 to produce methane, namely, the methanation process. There is extreme potential in methane serving as an energy carrier for the efficient usage of existing energy supply infrastructure (such as city gas pipes, liquefied natural gas power plants), and low-carbon heat use.
Using hydrogen power generation to provide value in electricity production as a regulated power supply source and increase generation capacity is expected. It seems that hydrogen power generation will play an effective role in decreasing carbon emissions and provide a regulated power supply and backup power source for extending renewable energy. Massive amounts of hydrogen will be consumed by hydrogen-based power generation, so its international supply chain development is very important. The cooperation of hydrogen with natural gas can be used in power generation systems. Initially, it will be used in existing natural gas power plants and in small-scale cogeneration systems to promote hydrogen diffusion. Another significant aspect is related to its long-term electricity storage for utilization in massive renewable energy oversupply without output control. Hydrogen will play an effective role in energy storage, especially in large-scale systems and on a long-term basis over multiple seasons. Therefore, hydrogen will have a significant impact and act as a key player in renewable energy in order to reduce carbon amounts in the electricity system. Further developments will lead people to use hydrogen in large-scale energy storage, transportation fuel, and power-to-gas applications, thus providing a clean and viable energy source that can be used for vehicles, households, larger buildings, etc. Additionally, hydrogen derived from seawater is a potentially game-changing solution and is heralded as an enabler of the Grand Transition into a cleaner future
[3].
1.2. Hydrogen from Semiconductor-Based Photocatalysis
Concerning the clean production of hydrogen, semiconductor-based photocatalysis seems to be a promising scheme. Hydrogen production through photocatalysis processes is similar to that of photovoltaic systems via the utilization of semiconductor materials such as TiO
2 [4]. The catalysis process according to the phase distribution of catalysts, reagents, and products, can be categorized into two kinds, homogeneous and heterogeneous; however, due to its ease of separation, heterogeneous catalysis is more preferred. A kind of heterogeneous catalysis that utilizes photon energy is known as semiconductor-based photocatalysis, in which a solid material is stimulated by exposure to light and generates an electron–hole pair. Subsequently, the photogenerated species take part in redox reactions. The first inspirational introduction of the photoelectrochemical cell dates back to 1972 by Honda and Fujishima, in which the water was split into oxygen and hydrogen using ultraviolet (UV) irradiation
[5]; since then, many studies have focused on the development and improvement of the photocatalysis process.
In these processes, the photogenerated carriers should migrate to the surface of the photocatalysts to engage in necessary electrochemical reactions. One of the most important challenges is the recombination of photogenerated charge carriers (electrons and holes), which, unfortunately, is preferable in terms of thermodynamic energy. This negatively impacts photocatalysis performance since a small number of photogenerated species can find a chance to migrate to the surface of the catalysts and become involved in redox reactions. Several strategies have been introduced to overcome the electron–hole recombination issue, including the inclusion of noble-metal co-catalysts
[6][7], cation/anion doping
[8][9], and the fabrication of heterojunctions between different semiconductors, which is also referred to as composite photocatalysis
[10][11].
Hierarchically ordered macro-mesoporous TiO2-graphene composite films show the enriched capacity of quickly adsorbing and photodegrading organic dyes. The assembling of interconnected macropores in mesoporous films has the following effects: (1) improves the mass transport through the film; (2) increases the accessible surface area of the thin film; (3) significantly enhances their photocatalytic activities; and (4) reduces the length of the mesopore channel. These structures are potentially suitable for wastewater treatment and air purification for removing organic pollutants and will provide an attractive opportunity for industrial applications.
Photocatalytic processes have the potential to develop renewable and eco-friendly energy carriers such as H
2 and O
2 by water splitting
[12][13]; they can also degrade various organic pollutants
[14][15][16][17][18], convert energy conversion, be used in material storage by photocatalytic CO
2 reduction
[19][20][21], synthesize organic substances
[14][22][23], and even reduce iron ore with hydrogen
[24]. In this regard, both energy issues and environmental pollution treatment can be economically solved by semiconductor-mediated photocatalysis procedures, and hydrogen, as an ideal green fuel, can be produced through the photocatalytic reduction of protons by the photoinduced electrons; hence, toxic organic pollutants can be degraded by utilization of multi-step photocatalytic oxidation reactions
[25]. Unfortunately, a single semiconductor cannot lead to quick recombination of photoinduced charge carriers because of the extensive exciton binding energy
[26][27]. Therefore, the fabrication of heterostructure photocatalysts, well-designed heterojunctions, and multi-component semiconductors is of eminent importance to maintain simultaneous extended light-harvesting range, efficient interfacial charge separation, and high redox potential. It was proven that realistic and intelligent selection of semiconductors and tailoring of well-suited energy band structures for the production of heterojunction photocatalysts should be fully considered to fulfill the above-mentioned factors
[28][29][30]. Until now, numerous strategies have been introduced to improve the photocatalytic performance of semiconductors for efficient hydrogen production, including homogenous and heterogenous methods, S-scheme, Z-scheme, and artificial photocatalysis
[31].
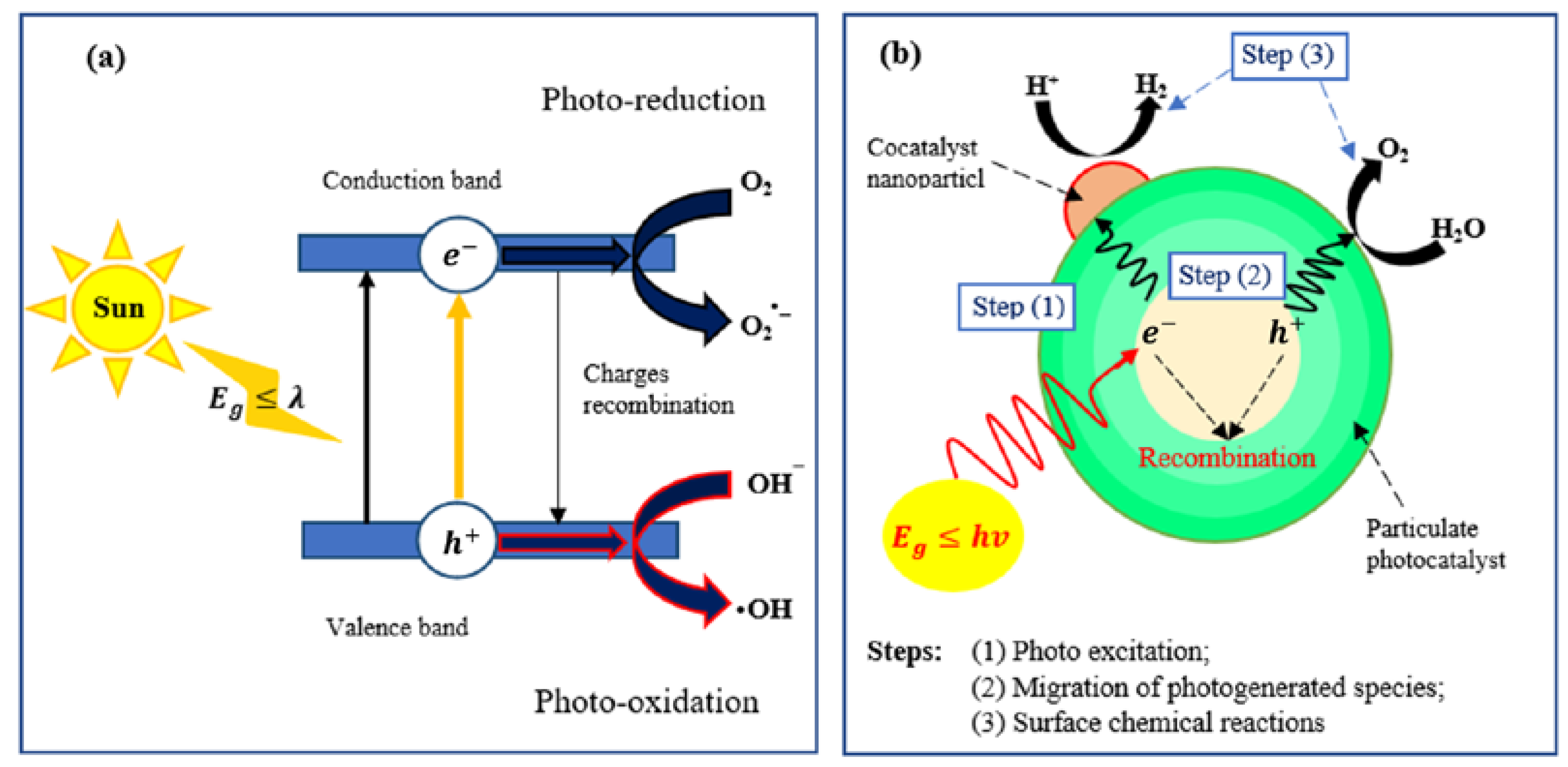
2. Semiconductor Photocatalysis
Semiconductor stimulation by photons with energies higher or equal to the semiconductor’s bandgap (E
g) leads to the promotion of an electron (e
−) from the valence band (VB) to the conduction band (CB), generating a hole (h
+). Both the excited state in the conduction band and holes in the valence band are thermodynamically and kinetically prone to recombine and spend the input energy through heat generation or light emission, as illustrated in
Figure 1a
[32]. By comparison, if electrons and holes migrate to the semiconductor’s surface without recombination, they can actively operate in electrochemical processes with species absorbed at the semiconductor surface. In this condition, photogenerated electrons act as reducing agents, and holes act as oxidizing agents. This redox capability of electron–hole pairs can be utilized in photocatalytic processes such as water/air remediation and hydrogen production.
Figure 1. Photocatalytic reactions in semiconductors: (a) photocatalysis process and (b) three basic steps of photocatalysis in semiconductors.
Photocatalytic reactions in semiconductors have three basic steps, as shown in
Figure 1b
[33]: (1) photon excitation, (2) migration of photogenerated species, and (3) surface chemical reactions. In these processes, both electrons and holes play a crucial role; photogenerated electrons play a substantial role in water reduction while holes assist in the oxidative decomposition of environmental pollutants. These photocatalytic reactions, via the cooperation of electrons and holes, lead to hydrogen production and CO
2 reduction to fabricate further reduced carbon species such as CO or methanol.
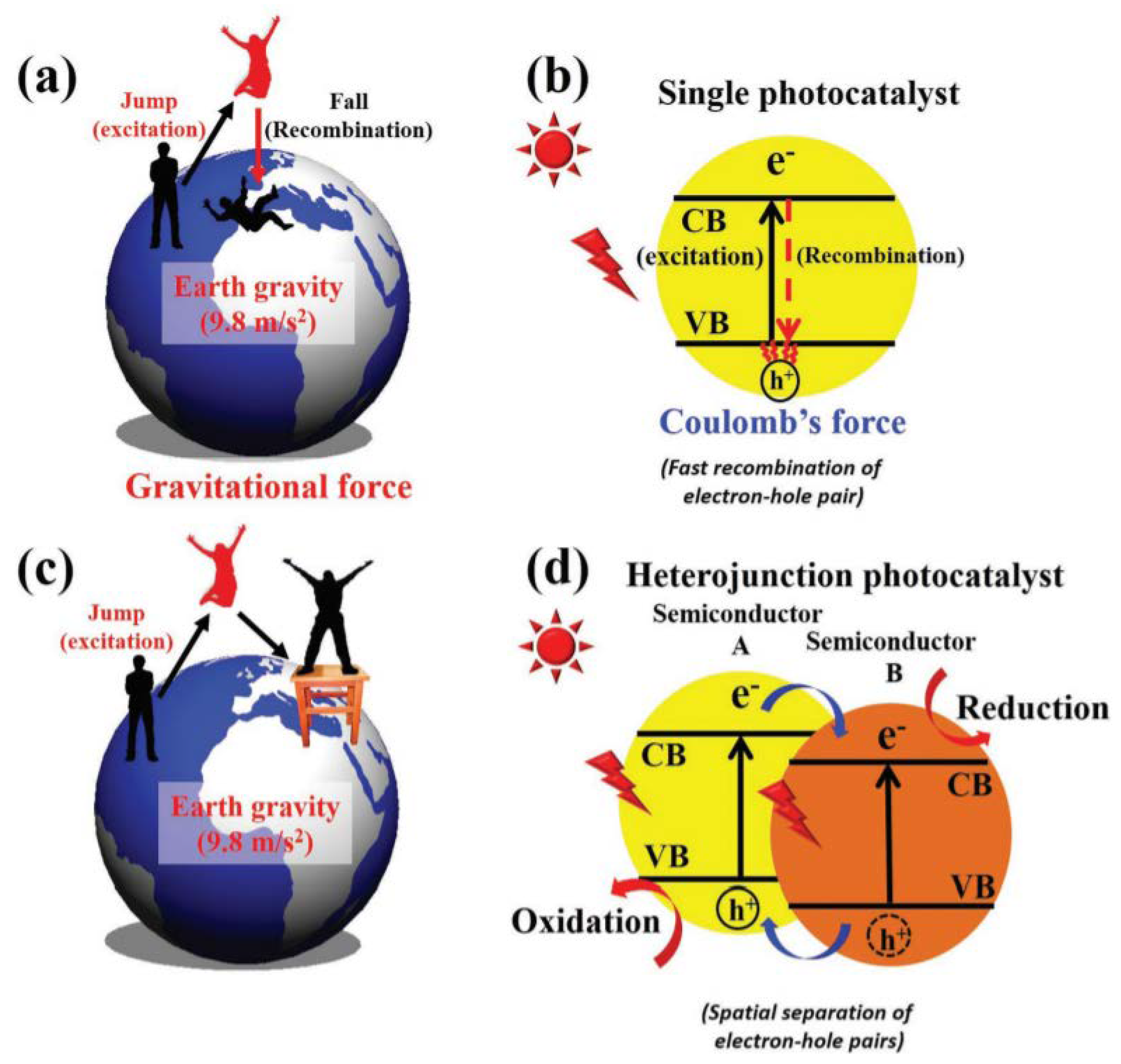
3. Semiconductor Heterojunctions
Heterojunctions are generated at the interface between different semiconductors, and they are utilized in the case of unequal bandgaps, discontinuities in the bandgap, or the existence of an abrupt barrier in a specific band
[34], as shown in
Figure 2.
Figure 2. Illustration of: (
a) the gravitational force effect on a jumping man; (
b) electron–hole recombination on a single photocatalyst; (
c) keeping a man off the ground using a stool; and (
d) electron–hole separation on a heterojunction photocatalyst, adapted from
[34].
When the electron (the man in
Figure 2a) jumps from the valence band (the ground in
Figure 2a) into the conduction band (the sky in
Figure 2a), it must immediately recombine with a hole (the ground in
Figure 2a) because of Coulomb attraction between the electron and the hole (the gravity of the Earth). Thus, in order to separate the electron–hole pairs that are photogenerated (keeping the man off the ground), the employment of a semiconductor B is needed (
Figure 2c,d) and the electron and hole pairs remain separated. The composite photocatalyst or heterojunction has been designed to solve the issues of maximum charge carrier recombination along with the minimum visible-light activity. Heterojunctions with proper design can considerably improve the separation of photogenerated electron–hole pairs; hence, they can readily move to the surface to become involved in subsequent electrochemical reactions
[35], as illustrated in
Figure 3.
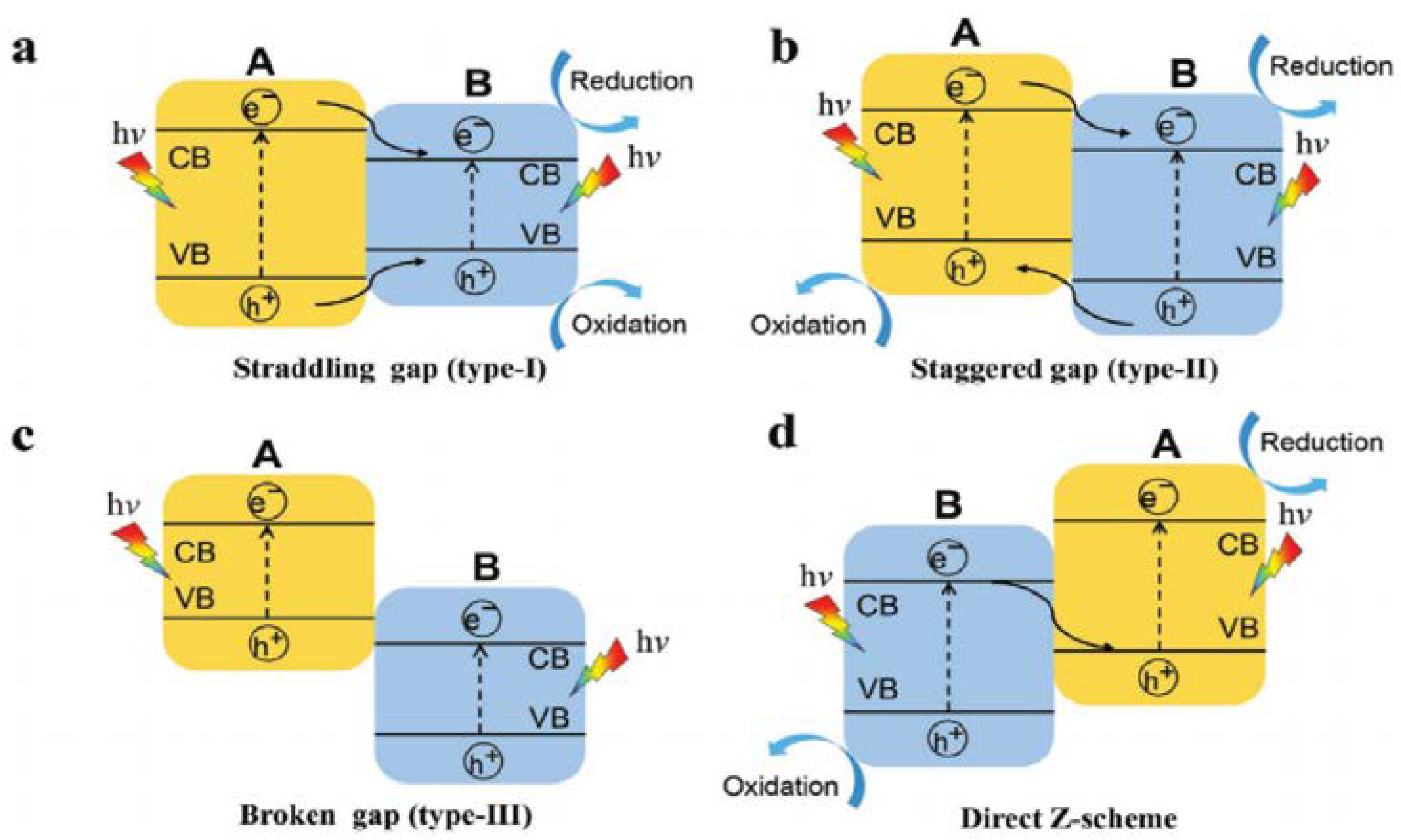
Figure 3. The electron–hole pairs’ migration under irradiation in various type of heterojunction photocatalysts: (
a) type-I, (
b) type-II, (
c) type-III, and (
d) direct Z-scheme, adapted from
[35].
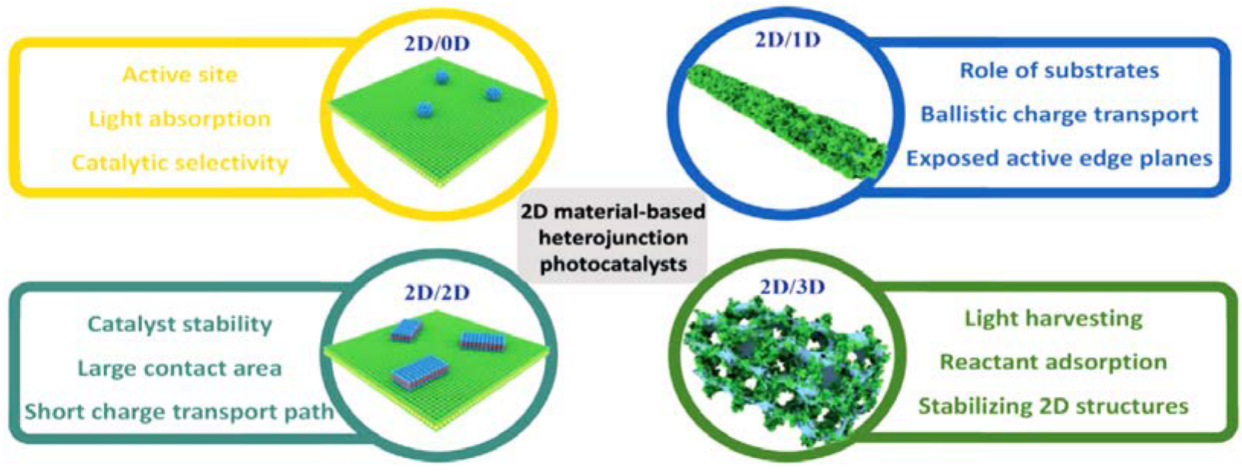
The unique physical and special chemical properties of 2D semiconductors make them an ideal option for fundamental photocatalytic research and potential commercial applications. Heterojunctions employment allows for the extension of the light adsorption interval, for the efficient separation of the carriers of energy that are produced, and for the improvement in the photocatalytic activity. Here, the specific design of heterojunctions is fundamental to the optimization of components’ functionalities and to the improvement of any synergic effect. As a matter of fact, 0D configurations allow for the activation of more active sites and the reduction in the migration distances of the charges. They can be designed using a structure having very versatile tunable bands in order to increase the catalytic activity. One-dimensional configurations act as very efficient charge transport from a ballistic point of view. In addition, one of their main actions is the suppression of potential agglomeration of 2D materials. The combination of 2D/2D heterojunctions allows for the increase in contact areas by increasing the charge transfer efficiency. Three-dimensional configurations allow for the increase in the efficiency of energy utilization by improving the transportation of the reacting compounds to the reaction sites. In addition, many improved properties can be obtained through the combination of different configurations, such as 2D/0D, 2D/1D, 2D/2D, and 2D/3D, by increasing the range of photocatalytic activities. Heterojunctions fabricated by two different semiconductors can be categorized into two major groups, known as p-n and non-p-n heterojunctions.
The Effect of Morphology in Heterojunctions
The morphology and structure of heterojunctions can substantially influence the activity of catalysts. In addition, the dimension and configuration can also affect the performance of heterojunction photocatalysts. It was shown that two-dimensional (2D) materials are among the proper candidates for photocatalysis applications because of their favorable structural and electronic properties, but they still need to be improved because of disadvantages such as electron–hole recombination and low redox ability. In this regard, wise and objective construction of 2D heterojunction photocatalysts with various arrangements and morphologies can improve the photocatalytic activity
[36];
Figure 4 shows the design of proficient 2D heterojunction photocatalysts with differing configurations, applications, and properties, each of which has its advantages and disadvantages.
Figure 4. The schematic illustration of 2D heterojunction photocatalysts with various configurations, applications, and properties, adapted from
[36].
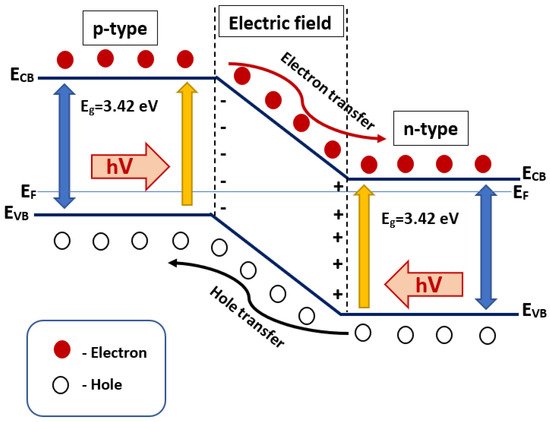
4. p-n Heterojunctions
In this type of heterojunction, two contacting semiconductors generate a p-n junction with a unique charge zone, enabling it to transmit electrons and holes in the reversed direction, as illustrated in
Figure 5. In this regard, holes are transmitted to the VB region of the p-type semiconductor, while electrons are transmitted to the CB region of the n-type semiconductor. The driving force for this electron–hole transfer is the electric field generated inside the space charge region (junction). During the exposure of p–n heterojunction to photons with equal or higher bandgap energies than those of the photocatalysts, the photogenerated electron–hole pairs can be separated instantly due to the formed electric field in the space charge region. In this condition, the holes are moved to the VB zone of the p-type and the electrons are transferred to the CB region of the n-type semiconductors. This type of p-n semiconductor has many advantages, including (i) very effective charge separation; (ii) accelerated charge transfer; (iii) increased charge carriers’ lifetime; and (iv) a regional separation of incompatible redox reactions in nano space
[37].
Figure 5. Schematic of p-n heterojunctions illustrating the energy band condition and separation of electron–hole pair.
According to the donor and acceptor characteristics, all semiconducting materials can be regarded either as a p- or n-type. If the p- and n-type semiconductors are coupled as shown in Figure 5 with suitable band gaps and band positions, they fabricate a form of p-n heterojunction composite. There are numerous varieties of p-n heterojunctions utilized in photocatalyst semiconductors, such as Cu2O-TiO2, NiO-TiO2, NiO-ZnO, CuO-ZnO, CuO-BiVO4, Bi2O3-Bi2WO6, and CaFe2O4-ZnO. It should be noted that the synthesis method is a crucial stage, and has a substantial impact on the mechanism and overall performance.
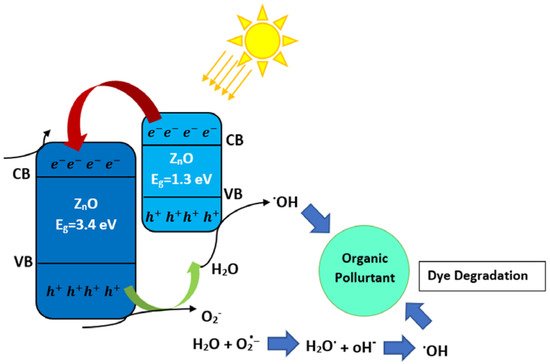
Photocatalytic decontamination is a promising procedure for environmental protection, with the ability to oxidize and mineralize numerous organic contaminants. Zinc oxide (ZnO), because of its favorable photoactivity, stability, availability, and nontoxic nature, is among the most interesting photocatalysts
[37]. Unfortunately, ZnO suffers from a very poor response to visible light and unfavorable recombination of photogenerated species; thus, the constructing heterojunctions having the suitable band edges can substantially overcome the ZnO shortcomings and improve its photocatalytic performance. It was reported that ZnO-based binary and multiple heterojunction/nanocomposite photocatalysts, such as hydrothermally fabricated ZnO/CuO heterostructures, exhibit the best photocatalytic activity
[38], as shown in
Figure 6.
Figure 6. Schematic of a photocatalytic mechanism of ZnO/CuO heterojunction.
Accordingly, Habibi-Yangjeh et al.
[39] synthesized a p-n heterojunction with ZnO/ZnBi
2O
4 nanocomposite to fabricate a durable visible-light-activated photocatalyst for organic pollutant degradation. The ZnO/ZnBi
2O
4 p-n heterojunction exhibits considerable photocatalytic ability compared to ZnO, with high throughput performance in the degradation of different pollutants. It should be mentioned that, in addition to photocatalytic applications of ZnO, it can also be used for antibacterial improvements due to toxicity mechanisms in ZnO, including Zn
2+ release, reactive oxygen species (ROS) generation, membrane dysfunction, and nanoparticle internalization into cells
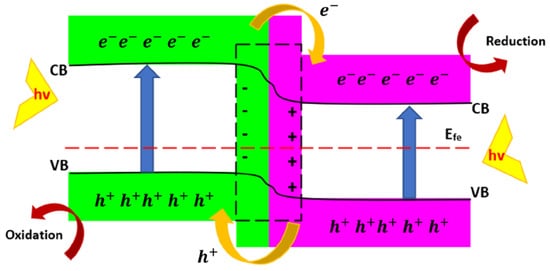
[40]. Although ZnO is considered one of the most traditional photocatalysts, numerous studies must be conducted for its improvement, particularly to enhance its visible-light response via bandgap narrowing. These include integration of ZnO with metallic and/or nonmetallic dopants, polymeric fabrications, dyes, and/or small bandgap semiconductors; heterojunction production between two or more semiconductors; and designing hierarchically structured photocatalysts with exposed reactive facets, as shown in
Figure 7 [41].
Figure 7. A simplified illustration of a p–n heterojunction construction.
Photocatalytic activity alongside photocatalytic recyclability of ZnO can be improved by the utilization of magnetic semiconductor materials. In the literature, numerous choices can be found for heterojunction construction, expanding the potential application of ZnO photocatalysts in practice, specifically in the environmental fields. A comprehensive study is required to investigate the variations in the electronic and lattice structure of the modified ZnO when it is doped/loaded with charged/uncharged elements and small bandgap semiconductors. In order to further comprehend the involved mechanism during photocatalytic reactions and construct efficient photoreactors, a modeling study is required for practical purposes.
This entry is adapted from the peer-reviewed paper 10.3390/en15093222