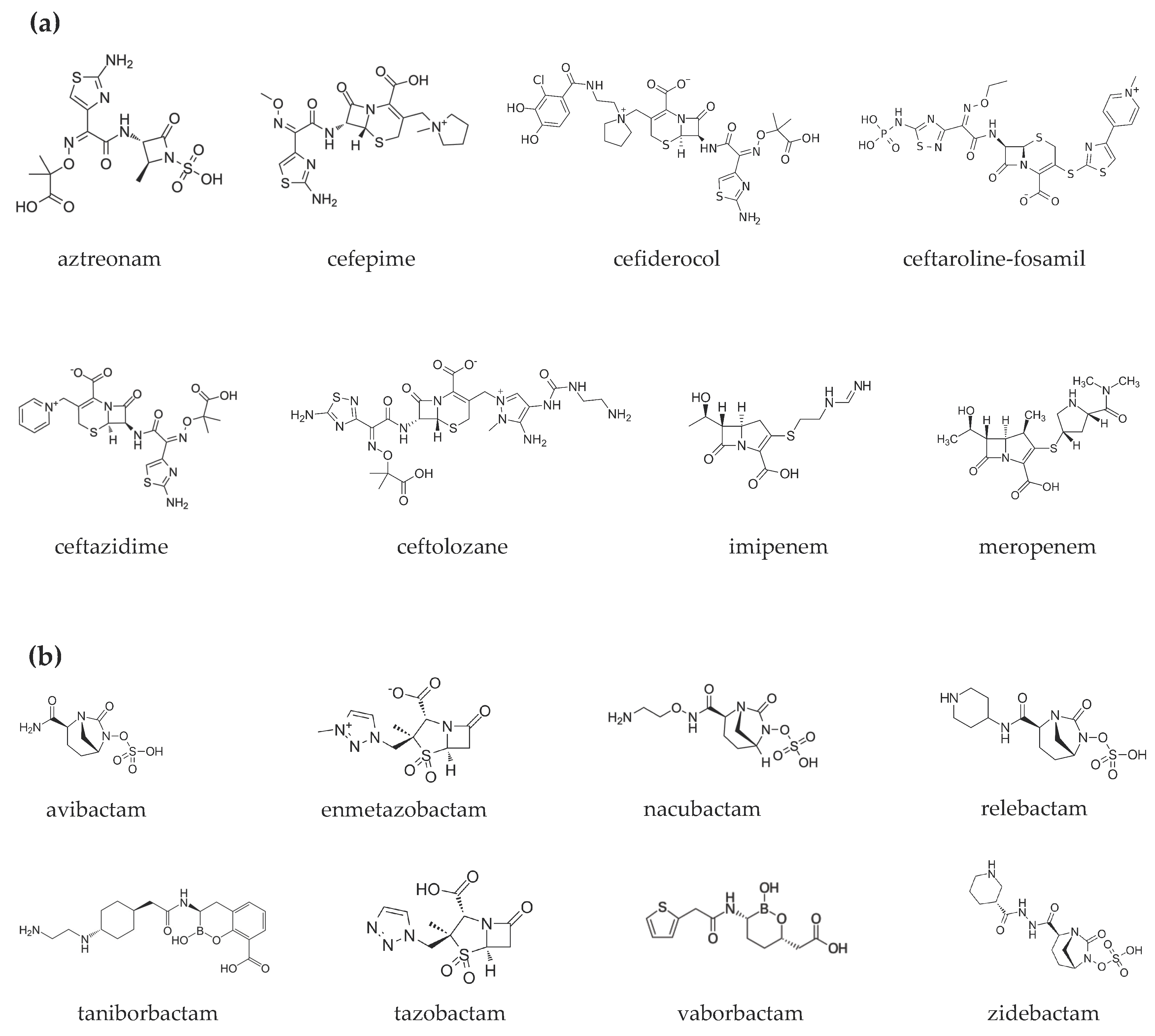
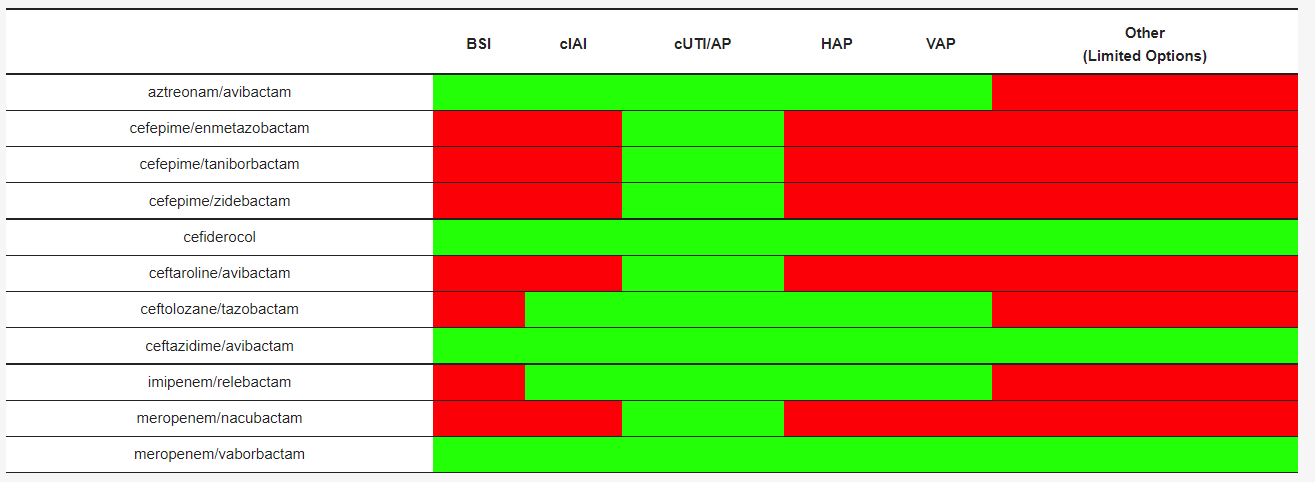
The epidemiology of infections sustained by multidrug-resistant Gram-negative bacteria is rapidly evolving. New drugs are available or are on the horizon. Most are combinations of a β-lactam and a β-lactamase inhibitor. One part is the antibiotic cefiderocol that has a peculiar antibacterial mechanism of action. Dispensing of such an armamentarium requires in-depth knowledge of their microbiological spectrum of activity, pharmacokinetic/pharmacodynamic (PK/PD) properties, and clinical study results. The following will describe the antibacterial strategy of aztreonam in combination with avibactam.
- Aztreonam/Avibactam
1. Introduction
2. Aztreonam/Avibactam

| DRUGS | PK/PD Index | T ½ (h) | Vd (L) | PB (%) | ELF/ Plasma (%) |
References |
|---|---|---|---|---|---|---|
| aztreonam/avibactam | 60% fT > MIC/ 50% fT > CT |
2.3–2.8/1.8–2.2 | 20/26 | 56/8 * | 30/30 | [6][8][11][12][13] |
| cefepime/ enmetazobactam |
60% fT > MIC/ 20–45% fT > CT |
2.1/** | 18.2/** | 16–19/** | 61/53 | [14][15][16] |
| cefepime/taniborbactam | 50% fT > MIC/ fAUC24/MIC |
2.1/4.7 * | 18.2/37.4 | 16–19/** | na | [16][17][18] |
| cefepime/zidebactam | 30% fT > MIC/ fAUC24/MIC |
2.0/1.9 | 15.4/17.4 | 20/< 15 | 39/38 | [19][20] |
| cefiderocol | ƒT/MIC ≥75% | 2.7 | 18 | 40–60 | 10–23 | [11][21][22] |
| ceftaroline-fosamil/ avibacatm |
40–50% fT > MIC/ f T > CT; fAUC |
2.4/2.0 * | 19.8/18 * | 20/8 * | 23/30 * | [8][23][24] |
| ceftolozane/ tazobactam |
35% fT > MIC/ % f T > CT |
3.5/2.5 | 13.5/18.2 | 21/30 | 61/63 | [25][26][27] |
| ceftazidime/avibactam | 50 % fT > MIC/ 40 % fT > CT |
2.0/2.0 | 14.3/15–25 | <10/5.7–8.2 | 52/42 | [8][11][28][29][30] |
| imipenem/relebactam | 6.5% fT > MIC/ fAUC24/MIC |
1/1.2 | 24.3/19 | 20/22 | 55/54 | [29][31][32] |
| meropenem/nacubactam | 40% fT > MIC/ fAUC24/MIC * |
1/2.6 * | 15–20/21.9 * | 2/2 * | na | [33] |
| meropenem/vaborbactam | 40% fT > MIC/ fAUC24/MIC * |
1.3/1.9 | 20.2/18.6 | 2/33 | 65/79 | [34][35][36][37] |
| Drugs | Recommended Dosage | Adjustment in RI | Authorized for Use in the European Union and by FDA |
References |
|---|---|---|---|---|
| aztreonam/ avibactam |
Not available | Not available | no | |
| cefepime/ enmetazobactam |
Not available | Not available | no | |
| cefepime/ taniborbactam |
Not available | Not available | no | |
| cefepime/ zidebactam |
Not available | Not available | no | |
| cefiderocol | Pneumonia: 2 g q 8 h (7 days) cUTI: 2 g q 8 h (7–14 days) |
CrCl ≥120 mL/min: 2 g q 6 h CrCl 60–120 mL/min: 2 g q 8 h CrCl 30–60 mL/min: 1.5 g q 8 h CrCl 15–30 mL/min: 1 g q 8 h CrCl <15 mL/min: 750 mg q 12 h |
yes | [24][38][39] |
| ceftaroline-fosamil/ avibactam |
Not available | no | ||
| ceftozolane/ tazobactam |
cIAI: 1.5–3 g q 8 h (4–5 days) Pneumonia: 3 g q 8 h (7 days) Bloodstream infection, skin and soft tissues: 1.5–3 g q 8 h cUTI: 1.5 g q 8 h |
CrCl >50 mL/min: 1.5 g q 8 h 3 g q 8 h CrCl 30–50 mL/min: 750 mg q 8 h 1.5 g q 8 h CrCl 15–29 mL/min: 375 mg q 8 h 750 mg q 8 h |
yes | [40][41][42][43][44] |
| ceftazidime/ avibactam |
cIAI: 2.5 g q 8 (4–5 days) Pneumonia: 2.5 g q h (7 days) cUTI: 2.5 g q 8 h (5–14 days) |
CrCl >50 mL/min: 2.5 g q 8 h CrCl 31–50 mL/min: 1.25 g q 8 h CrCl 16–30 mL/min: 0.94 g q 12 h CrCl 6–15 mL/min: 0.94 g q 24 h CrCl <5 mL/min: 0.94 g q 48 h |
yes | [30] |
| imipenem/ relebactam |
cIAI: 1.25 g q 6 h (4–7 days) Pneumonia: 1.25 g q 6 h (7 days) cUTI: 1.25 g q 6 h (5–14 days) |
CrCl ≥90 mL/min: 1.25 g q 6 h CrCl 60–89 mL/min: 1 g q 6 h CrCl 30–59 mL/min: 0.75 g q 6 h CrCl 15–29 mL/min: 0.5 g q 6 h CrCl <15 mL/min: 0.5 g q 6 h |
yes | [32][45][46] |
| meropenem/ vaborbactam |
cUTI: 4 g q 8 h (5–14 days) |
CrCl ≥50 mL/min: 4 g q 8 h CrCl 30–49 mL/min: 2 g q 8 h CrCl 15–29 mL/min: 2 g q 12 h CrCl <15 mL/min: 1 g q 12 h |
yes | [37][47][48][49][50] |
| meropenem/nacubactam | Not available | Not available | no |
This entry is adapted from the peer-reviewed paper 10.3390/ph15040463
References
- Tan, X.; Kim, H.S.; Baugh, K.; Huang, Y.; Kadiyala, N.; Wences, M.; Singh, N.; Wenzler, E.; Bulman, Z.P. Therapeutic Options for Metallo-β-Lactamase-Producing Enterobacterales. Infect. Drug Resist. 2021, 14, 125–142.
- Mauri, C.; Maraolo, A.E.; Di Bella, S.; Luzzaro, F.; Principe, L. The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases. Antibiotics 2021, 10, 1012.
- Sader, H.S.; Carvalhaes, C.G.; Arends, S.J.R.; Castanheira, M.; Mendes, R.E. Aztreonam/avibactam Activity against Clinical Isolates of Enterobacterales Collected in Europe, Asia and Latin America in 2019. J. Antimicrob. Chemother. 2021, 76, 659–666.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical Laboratory Standards Institute: Malvern, PA, USA, 2021.
- Aztreonam/Avibactam—List Results. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=aztreonam%2Favibactam&term=&cntry=&state=&city=&dist= (accessed on 5 February 2022).
- Cornely, O.A.; Cisneros, J.M.; Torre-Cisneros, J.; Rodríguez-Hernández, M.J.; Tallón-Aguilar, L.; Calbo, E.; Horcajada, J.P.; Queckenberg, C.; Zettelmeyer, U.; Arenz, D.; et al. Pharmacokinetics and Safety of Aztreonam/avibactam for the Treatment of Complicated Intra-Abdominal Infections in Hospitalized Adults: Results from the REJUVENATE Study. J. Antimicrob. Chemother. 2020, 75, 618–627.
- Falcone, M.; Menichetti, F.; Cattaneo, D.; Tiseo, G.; Baldelli, S.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Di Paolo, A.; Pai, M.P. Pragmatic Options for Dose Optimization of Ceftazidime/avibactam with Aztreonam in Complex Patients. J. Antimicrob. Chemother. 2021, 76, 1025–1031.
- Dimelow, R.; Wright, J.G.; MacPherson, M.; Newell, P.; Das, S. Population Pharmacokinetic Modelling of Ceftazidime and Avibactam in the Plasma and Epithelial Lining Fluid of Healthy Volunteers. Drugs R&D 2018, 18, 221–230.
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The “Old” and the “New” Antibiotics for MDR Gram-Negative Pathogens: For Whom, When, and How. Front. Public Health 2019, 7, 151.
- Di Paolo, A.; Gori, G.; Tascini, C.; Danesi, R.; Del Tacca, M. Clinical Pharmacokinetics of Antibacterials in Cerebrospinal Fluid. Clin. Pharmacokinet. 2013, 52, 511–542.
- Nichols, W.W.; Newell, P.; Critchley, I.A.; Riccobene, T.; Das, S. Avibactam Pharmacokinetic/Pharmacodynamic Targets. Antimicrob. Agents Chemother. 2018, 62, 02446-17.
- Luci, G.; Mattioli, F.; Falcone, M.; Di Paolo, A. Pharmacokinetics of Non-β-Lactam β-Lactamase Inhibitors. Antibiotics 2021, 10, 769.
- Ramsey, C.; MacGowan, A.P. A Review of the Pharmacokinetics and Pharmacodynamics of Aztreonam. J. Antimicrob. Chemother. 2016, 71, 2704–2712.
- Bernhard, F.; Odedra, R.; Sordello, S.; Cardin, R.; Franzoni, S.; Charrier, C.; Belley, A.; Warn, P.; Machacek, M.; Knechtle, P. Pharmacokinetics-Pharmacodynamics of Enmetazobactam Combined with Cefepime in a Neutropenic Murine Thigh Infection Model. Antimicrob. Agents Chemother. 2020, 64, e00078-20.
- Das, S.; Fitzgerald, R.; Ullah, A.; Bula, M.; Collins, A.M.; Mitsi, E.; Reine, J.; Hill, H.; Rylance, J.; Ferreira, D.M.; et al. Intrapulmonary Pharmacokinetics of Cefepime and Enmetazobactam in Healthy Volunteers: Towards New Treatments for Nosocomial Pneumonia. Antimicrob. Agents Chemother. 2020, 65, e01468-20.
- Okamoto, M.P.; Nakahiro, R.K.; Chin, A.; Bedikian, A. Cefepime Clinical Pharmacokinetics. Clin. Pharmacokinet. 1993, 25, 88–102.
- Abdelraouf, K.; Almarzoky Abuhussain, S.; Nicolau, D.P. In Vivo Pharmacodynamics of New-Generation β-Lactamase Inhibitor Taniborbactam (formerly VNRX-5133) in Combination with Cefepime against Serine-β-Lactamase-Producing Gram-Negative Bacteria. J. Antimicrob. Chemother. 2020, 75, 3601–3610.
- Dowell, J.A.; Dickerson, D.; Henkel, T. Safety and Pharmacokinetics in Human Volunteers of Taniborbactam (VNRX-5133), a Novel Intravenous β-Lactamase Inhibitor. Antimicrob. Agents Chemother. 2021, 65, e0105321.
- Lepak, A.J.; Zhao, M.; Andes, D.R. WCK 5222 (Cefepime/Zidebactam) Pharmacodynamic Target Analysis against Metallo-β-Lactamase Producing in the Neutropenic Mouse Pneumonia Model. Antimicrob. Agents Chemother. 2019, 63, e01648-19.
- Rodvold, K.A.; Gotfried, M.H.; Chugh, R.; Gupta, M.; Patel, A.; Chavan, R.; Yeole, R.; Friedland, H.D.; Bhatia, A. Plasma and Intrapulmonary Concentrations of Cefepime and Zidebactam Following Intravenous Administration of WCK 5222 to Healthy Adult Subjects. Antimicrob. Agents Chemother. 2018, 62, e00682-18.
- Saisho, Y.; Katsube, T.; White, S.; Fukase, H.; Shimada, J. Pharmacokinetics, Safety, and Tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-Negative Bacteria, in Healthy Subjects. Antimicrob. Agents Chemother. 2018, 62, e02163-17.
- Katsube, T.; Echols, R.; Wajima, T. Pharmacokinetic and Pharmacodynamic Profiles of Cefiderocol, a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69, S552–S558.
- Riccobene, T.A.; Su, S.F.; Rank, D. Single- and Multiple-Dose Study to Determine the Safety, Tolerability, and Pharmacokinetics of Ceftaroline Fosamil in Combination with Avibactam in Healthy Subjects. Antimicrob. Agents Chemother. 2013, 57, 1496–1504.
- Riccobene, T.A.; Pushkin, R.; Jandourek, A.; Knebel, W.; Khariton, T. Penetration of Ceftaroline into the Epithelial Lining Fluid of Healthy Adult Subjects. Antimicrob. Agents Chemother. 2016, 60, 5849–5857.
- Lepak, A.J.; Reda, A.; Marchillo, K.; Van Hecker, J.; Craig, W.A.; Andes, D. Impact of MIC Range for Pseudomonas Aeruginosa and Streptococcus Pneumoniae on the Ceftolozane in Vivo Pharmacokinetic/pharmacodynamic Target. Antimicrob. Agents Chemother. 2014, 58, 6311–6314.
- Xiao, A.J.; Caro, L.; Popejoy, M.W.; Huntington, J.A.; Kullar, R. PK/PD Target Attainment with Ceftolozane/Tazobactam Using Monte Carlo Simulation in Patients with Various Degrees of Renal Function, Including Augmented Renal Clearance and End-Stage Renal Disease. Infect. Dis. Ther. 2017, 6, 137–148.
- Nicolau, D.P.; De Waele, J.; Kuti, J.L.; Caro, L.; Larson, K.B.; Yu, B.; Gadzicki, E.; Zeng, Z.; Rhee, E.G.; Rizk, M.L. Pharmacokinetics and Pharmacodynamics of Ceftolozane/Tazobactam in Critically Ill Patients With Augmented Renal Clearance. Int. J. Antimicrob. Agents 2021, 57, 106299.
- Davido, B.; Fellous, L.; Lawrence, C.; Maxime, V.; Rottman, M.; Dinh, A. Ceftazidime-Avibactam and Aztreonam, an Interesting Strategy to Overcome β-Lactam Resistance Conferred by Metallo-β-Lactamases in Enterobacteriaceae and Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01008-17.
- Merdjan, H.; Rangaraju, M.; Tarral, A. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of Avibactam Alone and in Combination with Ceftazidime in Healthy Male Volunteers: Results of Two Randomized, Placebo-Controlled Studies. Clin. Drug Investig. 2015, 35, 307–317.
- Van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-Generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241.
- Rizk, M.L.; Rhee, E.G.; Jumes, P.A.; Gotfried, M.H.; Zhao, T.; Mangin, E.; Bi, S.; Chavez-Eng, C.M.; Zhang, Z.; Butterton, J.R. Intrapulmonary Pharmacokinetics of Relebactam, a Novel β-Lactamase Inhibitor, Dosed in Combination with Imipenem-Cilastatin in Healthy Subjects. Antimicrob. Agents Chemother. 2018, 62, e01411-17.
- Heo, Y.-A. Imipenem/Cilastatin/Relebactam: A Review in Gram-Negative Bacterial Infections. Drugs 2021, 81, 377–388.
- Mallalieu, N.L.; Winter, E.; Fettner, S.; Patel, K.; Zwanziger, E.; Attley, G.; Rodriguez, I.; Kano, A.; Salama, S.M.; Bentley, D.; et al. Safety and Pharmacokinetic Characterization of Nacubactam, a Novel β-Lactamase Inhibitor, Alone and in Combination with Meropenem, in Healthy Volunteers. Antimicrob. Agents Chemother. 2020, 64, e02229-19.
- Dhillon, S. Meropenem/Vaborbactam: A Review in Complicated Urinary Tract Infections. Drugs 2018, 78, 1259–1270.
- Wenzler, E.; Scoble, P.J. An Appraisal of the Pharmacokinetic and Pharmacodynamic Properties of Meropenem-Vaborbactam. Infect. Dis. Ther. 2020, 9, 769–784.
- Wenzler, E.; Gotfried, M.H.; Loutit, J.S.; Durso, S.; Griffith, D.C.; Dudley, M.N.; Rodvold, K.A. Meropenem-RPX7009 Concentrations in Plasma, Epithelial Lining Fluid, and Alveolar Macrophages of Healthy Adult Subjects. Antimicrob. Agents Chemother. 2015, 59, 7232–7239.
- Zhuang, L.; Yu, Y.; Wei, X.; Florian, J.; Jang, S.H.; Reynolds, K.S.; Wang, Y. Evaluation of Hemodialysis Effect on Pharmacokinetics of Meropenem/Vaborbactam in End-Stage Renal Disease Patients Using Modeling and Simulation. J. Clin. Pharmacol. 2020, 60, 1011–1021.
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and Safety of Cefiderocol or Best Available Therapy for the Treatment of Serious Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria (CREDIBLE-CR): A Randomised, Open-Label, Multicentre, Pathogen-Focused, Descriptive, Phase 3 Trial. Lancet Infect. Dis. 2021, 21, 226–240.
- Portsmouth, S.; Van Veenhuyzen, D.; Echols, R.; Machida, M.; Ferreira, J.C.A.; Ariyasu, M.; Tenke, P.; Nagata, T.D. Cefiderocol versus Imipenem-Cilastatin for the Treatment of Complicated Urinary Tract Infections Caused by Gram-Negative Uropathogens: A Phase 2, Randomised, Double-Blind, Non-Inferiority Trial. Lancet Infect. Dis. 2018, 18, 1319–1328.
- Wagenlehner, F.M.; Umeh, O.; Steenbergen, J.; Yuan, G.; Darouiche, R.O. Ceftolozane-Tazobactam Compared with Levofloxacin in the Treatment of Complicated Urinary-Tract Infections, Including Pyelonephritis: A Randomised, Double-Blind, Phase 3 Trial (ASPECT-cUTI). Lancet 2015, 385, 1949–1956.
- Kollef, M.H.; Nováček, M.; Kivistik, Ü.; Réa-Neto, Á.; Shime, N.; Martin-Loeches, I.; Timsit, J.-F.; Wunderink, R.G.; Bruno, C.J.; Huntington, J.A.; et al. Ceftolozane-Tazobactam versus Meropenem for Treatment of Nosocomial Pneumonia (ASPECT-NP): A Randomised, Controlled, Double-Blind, Phase 3, Non-Inferiority Trial. Lancet Infect. Dis. 2019, 19, 1299–1311.
- Dietch, Z.C.; Shah, P.M.; Sawyer, R.G. Advances in Intra-Abdominal Sepsis: What Is New? Curr. Infect. Dis. Rep. 2015, 17, 497.
- Hernández-Tejedor, A.; Merino-Vega, C.D.; Martín-Vivas, A.; Ruiz de Luna-González, R.; Delgado-Iribarren, A.; Gabán-Díez, Á.; Temprano-Gómez, I.; De la Calle-Pedrosa, N.; González-Jiménez, A.I.; Algora-Weber, A. Successful Treatment of Multidrug-Resistant Pseudomonas Aeruginosa Breakthrough Bacteremia with Ceftolozane/tazobactam. Infection 2017, 45, 115–117.
- Sousa Dominguez, A.; Perez-Rodríguez, M.T.; Nodar, A.; Martinez-Lamas, L.; Perez-Landeiro, A.; Crespo Casal, M. Successful Treatment of MDR Pseudomonas Aeruginosa Skin and Soft-Tissue Infection with Ceftolozane/tazobactam. J. Antimicrob. Chemother. 2017, 72, 1262–1263.
- Imipenem, Cilastatin Sodium, and Relebactam Monohydrate for the Treatment of Cancer Patients with Febrile Neutropenia. Available online: https://clinicaltrials.gov/ct2/show/NCT04983901 (accessed on 5 February 2022).
- Sims, M.; Mariyanovski, V.; McLeroth, P.; Akers, W.; Lee, Y.-C.; Brown, M.L.; Du, J.; Pedley, A.; Kartsonis, N.A.; Paschke, A. Prospective, Randomized, Double-Blind, Phase 2 Dose-Ranging Study Comparing Efficacy and Safety of Imipenem/cilastatin plus Relebactam with Imipenem/cilastatin Alone in Patients with Complicated Urinary Tract Infections. J. Antimicrob. Chemother. 2017, 72, 2616–2626.
- Kaye, K.S.; Bhowmick, T.; Metallidis, S.; Bleasdale, S.C.; Sagan, O.S.; Stus, V.; Vazquez, J.; Zaitsev, V.; Bidair, M.; Chorvat, E.; et al. Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection: The TANGO I Randomized Clinical Trial. JAMA 2018, 319, 788–799.
- Buckman, S.A.; Krekel, T.; Muller, A.E.; Mazuski, J.E. Ceftazidime-Avibactam for the Treatment of Complicated Intra-Abdominal Infections. Expert Opin. Pharmacother. 2016, 17, 2341–2349.
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.-F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-Avibactam versus Meropenem in Nosocomial Pneumonia, Including Ventilator-Associated Pneumonia (REPROVE): A Randomised, Double-Blind, Phase 3 Non-Inferiority Trial. Lancet Infect. Dis. 2018, 18, 285–295.
- Wagenlehner, F.M.; Sobel, J.D.; Newell, P.; Armstrong, J.; Huang, X.; Stone, G.G.; Yates, K.; Gasink, L.B. Ceftazidime-Avibactam Versus Doripenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: RECAPTURE, a Phase 3 Randomized Trial Program. Clin. Infect. Dis. 2016, 63, 754–762.
