Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
The epidemiology of infections sustained by multidrug-resistant Gram-negative bacteria is rapidly evolving. New drugs are available or are on the horizon. Most are combinations of a β-lactam and a β-lactamase inhibitor. One part is the antibiotic cefiderocol that has a peculiar antibacterial mechanism of action. Dispensing of such an armamentarium requires in-depth knowledge of their microbiological spectrum of activity, pharmacokinetic/pharmacodynamic (PK/PD) properties, and clinical study results.
- Aztreonam/Avibactam
1. Introduction
The epidemiology of infections sustained by multidrug-resistant Gram-negative bacteria is rapidly evolving. New drugs are available or are on the horizon. Most are combinations of a β-lactam and a β-lactamase inhibitor. One part is the antibiotic cefiderocol that has a peculiar antibacterial mechanism of action. Dispensing of such an armamentarium requires in-depth knowledge of their microbiological spectrum of activity, pharmacokinetic/pharmacodynamic (PK/PD) properties, and clinical study results. Here will will describe the aztreonam/avibactam.
2. Aztreonam/Avibactam
Aztreonam is an old antibiotic approved by the Food and Drug Administration (FDA) and the European regulatory authorities in 1986. Its clinical use was strongly limited by the spread of extended-spectrum β-lactamase (ESBL) and AmpC-type determinants. Of note, metallo-β-lactamases (MBLs) are able to hydrolyze all β-lactams except for the monobactam aztreonam. However, due to the frequent co-production of class A β-lactamases or AmpC-type determinants within MBL-producing Gram-negatives, aztreonam remains active only in one-third of cases [1]. For this reason, combining aztreonam with avibactam could represent a good antimicrobial strategy. A single product formulation of aztreonam/avibactam is currently under development in phase 3 studies for the treatment of MBL-sustained infections. Aztreonam/avibactam has antimicrobial activity against carbapenemase-producing Enterobacterales, P. aeruginosa (including isolates producing Klebsiella pneumoniae carbapenemase, KPC; Verona integron-encoded metallo-β-lactamase, VIM; imipenemase, IMP; New Delhi metallo-β-lactamase, NDM; and oxacillinase, OXA-48), and Stenotrophomonas maltophilia [2,3]. No antimicrobial activity has been reported against A. baumannii (no inhibition of A. baumannii OXA-type enzymes). Resistance in P. aeruginosa has been associated with impermeability (porin loss), the production of AmpC-type (Pseudomonas-derived cephalosporinase 1; PDC) variants, OXA enzymes (other than OXA-48), or hyperexpression of efflux systems, while resistance in Enterobacterales could be associated with a specific amino acid insertion (12 bp duplications) in PBP3 determinants causing a reduction in affinity for aztreonam [2] (Table 1). For antimicrobial susceptibility testing purpose, the concentration of avibactam is fixed at 4 mg/L [4]. No clinical breakpoint (CLSI, EUCAST, or FDA) has been approved for this combination. An EUCAST epidemiological cut-off (ECOFF) value has not been assigned.

Figure 1. Chemical structures of (a) β-lactams and (b) β-lactamase inhibitors.
Table 1. Microbiological targets.

Currently, seven clinical trials on aztreonam/avibactam are registered: five are completed and two are recruiting. The efficacy of the combination is being tested in patients with bloodstream infections (BSIs), complicated intra-abdominal infections (cIAIs), complicated urinary tract infections (cUTIs), hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP). In contrast, aztreonam/avibactam safety is more generally being evaluated in patients with serious or complicated bacterial infections [5]. Recently, a phase 2 trial was published: 34 patients with cIAIs were treated for 5–14 days with aztreonam/avibactam + metronidazole. No patients had either ESBL or MBL-positive isolates. Patients were divided into three cohorts: (1) 500/137 mg, followed by 1500/410 mg every 6 h; (2) 500/167 mg, followed by 1500/500 mg every 6 h; and (3) extension of exposure at the higher dose regimen. The most common adverse events were hepatic enzyme increases (26%) and diarrhea (15%). Clinical cure rates at the test-of-cure visit were 59% overall [6]. Data from this study supported the regimen selected for the phase 3 trial (500/167 mg, followed by 1500/500 mg every 6 h) (Table 2).
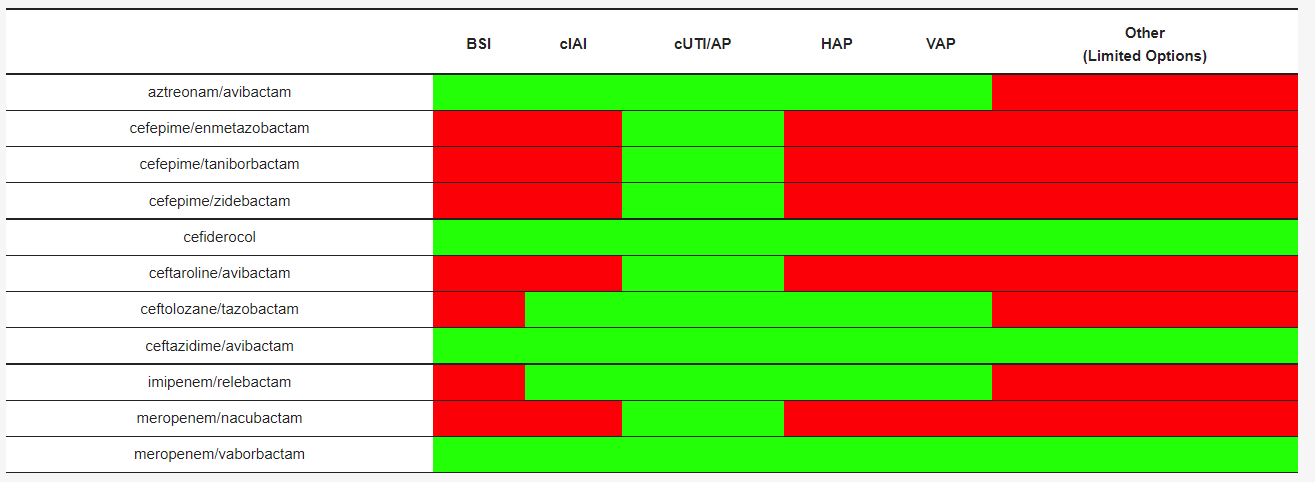
Table 2. Clinical settings investigated or under investigation for each compound.
In the recently published phase 2 clinical trial, aztreonam showed, in the first cohort, a geometric mean volume of distribution (Vd) of 20.0 L, 16.9% (geometric coefficient of variance) and a clearance (Cl) of 6.4 L/h (35.4%), while for avibactam, a Vd of 26.0 L (22.0%) and a Cl of 10.1 L/h (42.6%) was described. Similar data were obtained in the other two cohorts [6]. In patients with cIAI, avibactam’s Cl was lower, while avibactam and aztreonam Vd were higher than in healthy volunteers, as expected in critical patients, due to changes in protein levels, extracellular fluids, and blood volume [6], as already described for avibactam Vd in critical patients with comorbidities and burns [7]. Further PK studies on the combination have not yet been conducted, despite clinical experiences in various infections [2]. Studies on avibactam show that the drug diffuses into epithelial lung fluid (ELF) with concentrations around 30% of those in plasma [8,9]. Instead, the blood–brain barrier represents an obstacle to the diffusion [10] (Table 3 and Table 4).
Table 3. Pharmacokinetic parameters of β-lactams/β-lactamase inhibitors and cefiderocol. The concentrations of β-lactams and β-lactamase inhibitors were determined using liquid chromatography–tandem mass spectrometry.
| DRUGS | PK/PD Index | T ½ (h) | Vd (L) | PB (%) | ELF/ Plasma (%) |
References |
|---|---|---|---|---|---|---|
| aztreonam/avibactam | 60% fT > MIC/ 50% fT > CT |
2.3–2.8/1.8–2.2 | 20/26 | 56/8 * | 30/30 | [6,8,11,12,13] |
| cefepime/ enmetazobactam |
60% fT > MIC/ 20–45% fT > CT |
2.1/** | 18.2/** | 16–19/** | 61/53 | [14,15,16] |
| cefepime/taniborbactam | 50% fT > MIC/ fAUC24/MIC |
2.1/4.7 * | 18.2/37.4 | 16–19/** | na | [16,17,18] |
| cefepime/zidebactam | 30% fT > MIC/ fAUC24/MIC |
2.0/1.9 | 15.4/17.4 | 20/< 15 | 39/38 | [19,20] |
| cefiderocol | ƒT/MIC ≥75% | 2.7 | 18 | 40–60 | 10–23 | [11,21,22] |
| ceftaroline-fosamil/ avibacatm |
40–50% fT > MIC/ f T > CT; fAUC |
2.4/2.0 * | 19.8/18 * | 20/8 * | 23/30 * | [8,23,24] |
| ceftolozane/ tazobactam |
35% fT > MIC/ % f T > CT |
3.5/2.5 | 13.5/18.2 | 21/30 | 61/63 | [25,26,27] |
| ceftazidime/avibactam | 50 % fT > MIC/ 40 % fT > CT |
2.0/2.0 | 14.3/15–25 | <10/5.7–8.2 | 52/42 | [8,11,28,29,30] |
| imipenem/relebactam | 6.5% fT > MIC/ fAUC24/MIC |
1/1.2 | 24.3/19 | 20/22 | 55/54 | [29,31,32] |
| meropenem/nacubactam | 40% fT > MIC/ fAUC24/MIC * |
1/2.6 * | 15–20/21.9 * | 2/2 * | na | [33] |
| meropenem/vaborbactam | 40% fT > MIC/ fAUC24/MIC * |
1.3/1.9 | 20.2/18.6 | 2/33 | 65/79 | [34,35,36,37] |
Table 4. Recommended dosages and dose adjustment in renal insufficiency.
| Drugs | Recommended Dosage | Adjustment in RI | Authorized for Use in the European Union and by FDA |
References |
|---|---|---|---|---|
| aztreonam/ avibactam |
Not available | Not available | no | |
| cefepime/ enmetazobactam |
Not available | Not available | no | |
| cefepime/ taniborbactam |
Not available | Not available | no | |
| cefepime/ zidebactam |
Not available | Not available | no | |
| cefiderocol | Pneumonia: 2 g q 8 h (7 days) cUTI: 2 g q 8 h (7–14 days) |
CrCl ≥120 mL/min: 2 g q 6 h CrCl 60–120 mL/min: 2 g q 8 h CrCl 30–60 mL/min: 1.5 g q 8 h CrCl 15–30 mL/min: 1 g q 8 h CrCl <15 mL/min: 750 mg q 12 h |
yes | [24,38,39] |
| ceftaroline-fosamil/ avibactam |
Not available | no | ||
| ceftozolane/ tazobactam |
cIAI: 1.5–3 g q 8 h (4–5 days) Pneumonia: 3 g q 8 h (7 days) Bloodstream infection, skin and soft tissues: 1.5–3 g q 8 h cUTI: 1.5 g q 8 h |
CrCl >50 mL/min: 1.5 g q 8 h 3 g q 8 h CrCl 30–50 mL/min: 750 mg q 8 h 1.5 g q 8 h CrCl 15–29 mL/min: 375 mg q 8 h 750 mg q 8 h |
yes | [40,41,42,43,44] |
| ceftazidime/ avibactam |
cIAI: 2.5 g q 8 (4–5 days) Pneumonia: 2.5 g q h (7 days) cUTI: 2.5 g q 8 h (5–14 days) |
CrCl >50 mL/min: 2.5 g q 8 h CrCl 31–50 mL/min: 1.25 g q 8 h CrCl 16–30 mL/min: 0.94 g q 12 h CrCl 6–15 mL/min: 0.94 g q 24 h CrCl <5 mL/min: 0.94 g q 48 h |
yes | [30] |
| imipenem/ relebactam |
cIAI: 1.25 g q 6 h (4–7 days) Pneumonia: 1.25 g q 6 h (7 days) cUTI: 1.25 g q 6 h (5–14 days) |
CrCl ≥90 mL/min: 1.25 g q 6 h CrCl 60–89 mL/min: 1 g q 6 h CrCl 30–59 mL/min: 0.75 g q 6 h CrCl 15–29 mL/min: 0.5 g q 6 h CrCl <15 mL/min: 0.5 g q 6 h |
yes | [32,45,46] |
| meropenem/ vaborbactam |
cUTI: 4 g q 8 h (5–14 days) |
CrCl ≥50 mL/min: 4 g q 8 h CrCl 30–49 mL/min: 2 g q 8 h CrCl 15–29 mL/min: 2 g q 12 h CrCl <15 mL/min: 1 g q 12 h |
yes | [37,47,48,49,50] |
| meropenem/nacubactam | Not available | Not available | no |
Abbreviations: CrCl = creatinine clearance, cIAI = complicated intra-abdominal tract infection; cUTI = complicated urinary tract infection; RI = renal insufficiency; FDA = US Food and Drug administration.
This entry is adapted from the peer-reviewed paper 10.3390/ph15040463
This entry is offline, you can click here to edit this entry!
