The Y chromosome is one of the sex chromosomes found in males of animals of different taxa, including insects and mammals. Among all chromosomes, the Y chromosome is characterized by a unique chromatin landscape undergoing dynamic evolutionary change. Being entirely heterochromatic, the Y chromosome as a rule preserves few functional genes, but is enriched in tandem repeats and transposons. Due to difficulties in the assembly of the highly repetitive Y chromosome sequence, deep analyses of Y chromosome evolution, structure, and functions are limited to a few species, one of them being Drosophila melanogaster. Here we survey comparative evolutionary history of the fly and human Y chromosomes, peculiarities of transcription of giant genes, such as genes, encoding fertility factors in Drosophila, differential expression of sex-linked rDNA loci, and functions of Y-linked piRNA clusters ensuring sex-specific piRNA silencing. Our comparative analysis will provide further insight into the properties of the Y chromosomes in both insects and mammals.
- Drosophila
- Y chromosome
- piRNA pathway
- rDNA
- intron gigantism
- azoospermia
- transposable elements
1. Introduction
2. Comparative Evolutionary History of the Fly and Human Y Chromosomes
2.1. Y Chromosome Differentiation and Functions in Flies
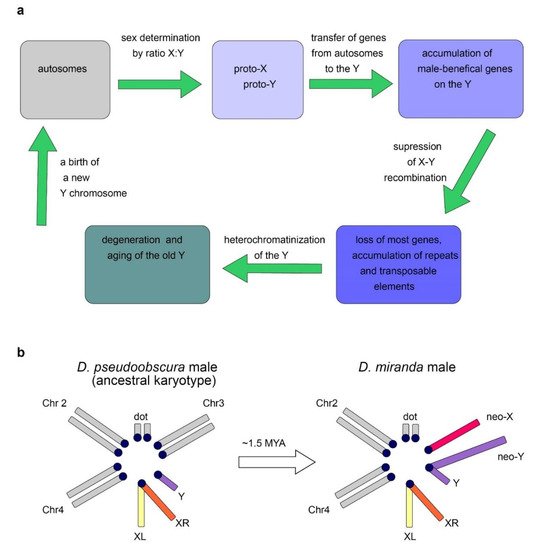
2.2. Origin of the Y Chromosome in Mammals and Sex Determination
2.3. Evolutionary Factors and Forces Determining the Structure and Functional Specialization of the Y Chromosome
2.4. Dosage Compensation System Contributes to Y-Linked Gene Maintenance
2.5. Convergent Nature of the Evolution of Y Chromosomes
3. Advances in the Study of Transcription of Y-linked Fly Giant Genes with the Application of this Knowledge in Undestanding Duchenne Muscular Dystrophy
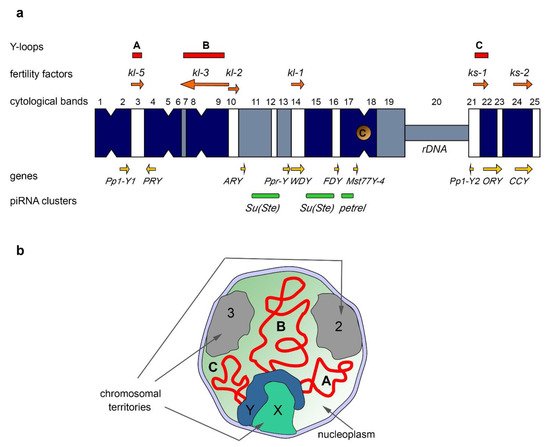
3.1. Fertility Factors and Y-Loop Formation in Drosophila
3.2. Intron Gigantism in Humans
4. New Discoveries in the Field of Differential Expression of rDNA Loci
4.1. Nucleolar Dominance as a Widespread Phenomenon
4.2. Y-Based Nucleolar Dominance in D. melanogaster Males
4.3. Non-Random Segregation of Sister Chromatids of Sex Chromosomes in Drosophila
4.4. Differential Expression of rDNA Loci in Human
5. Current Undestanding of Drosophila Y Chromosome Contribution in piRNA Biogenesis and Functioning of piRNA-Clusters
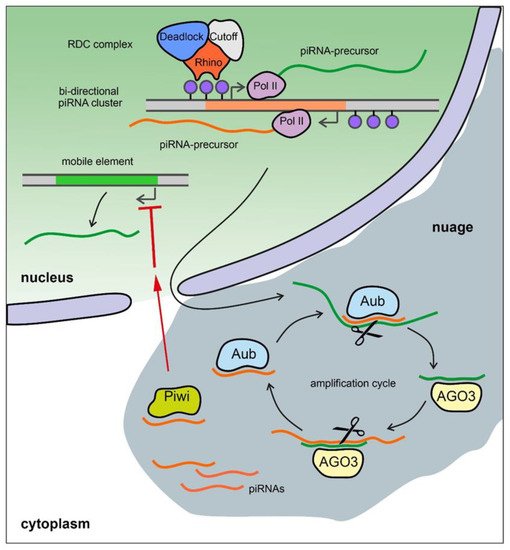
5.1. Brief Description of the piRNA System
5.2. The Y Chromosome as a Major piRNA-Producing Genomic Region in the Fly Testes
5.3. The Y Chromosome in Other Species as a Source of piRNAs
5.4. The Y Chromosome and TEs
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms23084184
References
- Bonaccorsi, S.; Lohe, A. Fine mapping of satellite DNA sequences along the Y chromosome of Drosophila melanogaster: Relationships between satellite sequences and fertility factors. Genetics 1991, 129, 177–189.
- Hoskins, R.A.; Carlson, J.W.; Wan, K.H.; Park, S.; Mendez, I.; Galle, S.E.; Booth, B.W.; Pfeiffer, B.D.; George, R.A.; Svirskas, R.; et al. The Release 6 reference sequence of the Drosophila melanogaster genome. Genome Res. 2015, 25, 445–458.
- Carvalho, A.B.; Vicoso, B.; Russo, C.A.; Swenor, B.; Clark, A.G. Birth of a new gene on the Y chromosome of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2015, 112, 12450–12455.
- Chang, C.H.; Larracuente, A.M. Heterochromatin-Enriched Assemblies Reveal the Sequence and Organization of the Drosophila melanogaster Y Chromosome. Genetics 2019, 211, 333–348.
- Chang, C.H.; Gregory, L.E.; Gordon, K.E.; Meiklejohn, C.D.; Larracuente, A.M. Unique structure and positive selection promote the rapid divergence of Drosophila Y chromosomes. eLife 2022, 11, e75795.
- Piergentili, R. Multiple roles of the Y chromosome in the biology of Drosophila melanogaster. Sci. World J. 2010, 10, 1749–1767.
- Chen, P.; Kotov, A.A.; Godneeva, B.K.; Bazylev, S.S.; Olenina, L.V.; Aravin, A.A. piRNA-mediated gene regulation and adaptation to sex-specific transposon expression in D. melanogaster male germline. Genes Dev. 2021, 35, 914–935.
- Salz, H.K.; Erickson, J.W. Sex determination in Drosophila: The view from the top. Fly 2010, 4, 60–70.
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Tree of Sex Consortium. Sex determination: Why so many ways of doing it? PLoS Biol. 2014, 12, e1001899.
- Carvalho, A.B.; Koerich, L.B.; Clark, A.G. Origin and evolution of Y chromosomes: Drosophila tales. Trends Genet. 2009, 25, 270–277.
- Vicoso, B.; Bachtrog, D. Numerous transitions of sex chromosomes in Diptera. PLoS Biol. 2015, 13, e1002078.
- Mahajan, S.; Bachtrog, D. Convergent evolution of Y chromosome gene content in flies. Nat. Commun. 2017, 8, 785.
- Koerich, L.B.; Wang, X.; Clark, A.G.; Carvalho, A.B. Low conservation of gene content in the Drosophila Y chromosome. Nature 2008, 456, 949–951.
- Bachtrog, D.; Mahajan, S.; Bracewell, R. Massive gene amplification on a recently formed Drosophila Y chromosome. Nat. Ecol. Evol. 2019, 3, 1587–1597.
- Bachtrog, D.; Charlesworth, B. Reduced adaptation of a non-recombining neo-Y chromosome. Nature 2002, 416, 323–326.
- Bachtrog, D. The Y Chromosome as a Battleground for Intragenomic Conflict. Trends Genet. 2020, 36, 510–522.
- Carvalho, A.B.; Clark, A.G. Y chromosome of D. pseudoobscura is not homologous to the ancestral Drosophila Y. Science 2005, 307, 108–110.
- Ricchio, J.; Uno, F.; Carvalho, A.B. New Genes in the Drosophila Y Chromosome: Lessons from D. willistoni. Genes 2021, 12, 1815.
- Ohno, S. Sex Chromosomes and Sex-Linked Genes; Monographs on Endocrinology; Springer: Berlin/Heidelberg, Germany, 1967.
- Potrzebowski, L.; Vinckenbosch, N.; Marques, A.C.; Chalmel, F.; Jégou, B.; Kaessmann, H. Chromosomal gene movements reflect the recent origin and biology of therian sex chromosomes. PLoS Biol. 2008, 6, e80.
- Veyrunes, F.; Waters, P.D.; Miethke, P.; Rens, W.; McMillan, D.; Alsop, A.E.; Grützner, F.; Deakin, J.E.; Whittington, C.M.; Schatzkamer, K.; et al. Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Res. 2008, 18, 965–973.
- Subrini, J.; Turner, J. Y chromosome functions in mammalian spermatogenesis. eLife 2021, 10, e67345.
- Foster, J.W.; Graves, J.A. An SRY-related sequence on the marsupial x chromosome: Implications for the evolution of the mammalian testis-determining gene. Proc. Natl. Acad. Sci. USA 1994, 91, 1927–1931.
- Graves, J.A. Evolution of the mammalian Y chromosome and sex-determining genes. J. Exp. Zool. 1998, 281, 472–481.
- Larson, E.L.; Kopania, E.E.K.; Good, J.M. Spermatogenesis and the evolution of mammalian sex chromosomes. Trends Genet. 2018, 34, 722–732.
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118, e2020909118.
- Rice, W.R. The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced recombination between primitive sex chromosomes. Evolution 1987, 41, 911–914.
- Rice, W.R. Genetic hitchhiking and the evolution of reduced genetic activity of the Y sex chromosome. Genetics 1987, 116, 161–167.
- Bachtrog, D. Y-chromosome evolution: Emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 2013, 14, 113–124.
- Charlesworth, B.; Charlesworth, D. The degeneration of Y chromosomes. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2000, 355, 1563–1572.
- Bellott, D.W.; Hughes, J.F.; Skaletsky, H.; Brown, L.G.; Pyntikova, T.; Cho, T.J.; Koutseva, N.; Zaghlul, S.; Graves, T.; Rock, S.; et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 2014, 508, 494–499.
- Lahn, B.T.; Page, D.C. Functional coherence of the human Y chromosome. Science 1997, 278, 675–680.
- Vogt, P.H. AZF deletions and Y chromosomal haplogroups: History and update based on sequence. Hum. Reprod. Update 2005, 11, 319–336.
- Kamp, C.; Huellen, K.; Fernandes, S.; Sousa, M.; Schlegel, P.N.; Mielnik, A.; Kleiman, S.; Yavetz, H.; Krause, W.; Küpker, W.; et al. High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol. Hum. Reprod. 2001, 7, 987–994.
- Lardone, M.C.; Parodi, D.A.; Valdevenito, R.; Ebensperger, M.; Piottante, A.; Madariaga, M.; Smith, R.; Pommer, R.; Zambrano, N.; Castro, A. Quantification of DDX3Y, RBMY1, DAZ and TSPY mRNAs in testes of patients with severe impairment of spermatogenesis. Mol. Hum. Reprod. 2007, 13, 705–712.
- Kotov, A.A.; Olenkina, O.M.; Godneeva, B.K.; Adashev, V.E.; Olenina, L.V. Progress in understanding the molecular functions of DDX3Y (DBY) in male germ cell development and maintenance. Biosci. Trends 2017, 11, 46–53.
- Bhowmick, B.K.; Satta, Y.; Takahata, N. The origin and evolution of human ampliconic gene families and ampliconic structure. Genome Res. 2007, 17, 441–450.
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825–837.
- Betrán, E.; Demuth, J.P.; Williford, A. Why chromosome palindromes? Int. J. Evol. Biol. 2012, 2012, 207958.
- Bonito, M.; D’Atanasio, E.; Ravasini, F.; Cariati, S.; Finocchio, A.; Novelletto, A.; Trombetta, B.; Cruciani, F. New insights into the evolution of human Y chromosome palindromes through mutation and gene conversion. Hum. Mol. Genet. 2021, 30, 2272–2285.
- Lange, J.; Skaletsky, H.; van Daalen, S.K.; Embry, S.L.; Korver, C.M.; Brown, L.G.; Oates, R.D.; Silber, S.; Repping, S.; Page, D.C. Isodicentric Y chromosomes and sex disorders as byproducts of homologous recombination that maintains palindromes. Cell 2009, 138, 855–869.
- Hughes, J.F.; Page, D.C. The Biology and Evolution of Mammalian Y Chromosomes. Annu. Rev. Genet. 2015, 49, 507–527.
- Traut, W.; Winking, H. Meiotic chromosomes and stages of sex chromosome evolution in fish: Zebrafish, platyfish and guppy. Chromosome Res. 2001, 9, 659–672.
- Graves, J.A. Sex chromosome specialization and degeneration in mammals. Cell 2006, 124, 901–914.
- Blackmon, H.; Ross, L.; Bachtrog, D. Sex Determination, Sex Chromosomes, and Karyotype Evolution in Insects. J. Hered. 2017, 108, 78–93.
- Griffin, D.K. Is the Y chromosome disappearing?—Both sides of the argument. Chromosome Res. 2012, 20, 35–45.
- Fisher, E.; Scambler, P. Human haploinsufficiency—One for sorrow, two for joy. Nat. Genet. 1994, 7, 5–7.
- Johnson, A.F.; Nguyen, H.T.; Veitia, R.A. Causes and effects of haploinsufficiency. Biol. Rev. 2019, 94, 1774–1785.
- Zug, R. Developmental disorders caused by haploinsufficiency of transcriptional regulators: A perspective based on cell fate determination. Biol. Open. 2022, 11, bio058896.
- Carrel, L.; Brown, C.J. When the Lyon(ized chromosome) roars: Ongoing expression from an inactive X chromosome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160355.
- Fang, H.; Disteche, C.M.; Berletch, J.B. X Inactivation and Escape: Epigenetic and Structural Features. Front. Cell Dev. Biol. 2019, 7, 219.
- Cortez, D.; Marin, R.; Toledo-Flores, D.; Froidevaux, L.; Liechti, A.; Waters, P.D.; Grützner, F.; Kaessmann, H. Origins and functional evolution of Y chromosomes across mammals. Nature 2014, 508, 488–493.
- Hook, E.B.; Warburton, D. Turner syndrome revisited: Review of new data supports the hypothesis that all viable 45, X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum. Genet. 2014, 133, 417–424.
- Gravholt, C.H.; Viuff, M.H.; Brun, S.; Stochholm, K.; Andersen, N.H. Turner syndrome: Mechanisms and management. Nat. Rev. Endocrinol. 2019, 15, 601–614.
- Stern, C. Untersuchungen über Aberrationen desY-chromosoms von Drosophila melanogaster. Zeitschrift für Induktive Abstammungs-und Vererbungslehre 1929, 51, 253–353. (In German)
- Shen, T.H. Zytologische Untersuchungen über Sterilität bei Männchen von Drosophila melanogaster und bei F1 Männchen der Kreuzung zwischen D. simulans-Weibchen und D. melanogaster-Männchen. Zeitschrift für Zellforschung und Mikroskopische Anatomie 1932, 15, 547–580. (In German)
- Brosseau, G.E. Genetic analysis of the male fertility factors on the Y chromosome of Drosophila melanogaster. Genetics 1960, 45, 257–274.
- Ayles, G.B.; Sanders, T.; Kiefer, B.; Suzuki, D. Temperature sensitive mutations in Drosophila melanogaster: XI. Male sterile mutants of the Y chromosome. Dev. Biol. 1973, 32, 239–257.
- Hardy, R.W.; Tokuyasu, K.T.; Lindsley, D.L. Analysis of spermatogenesis in Drosophila melanogaster bearing deletions for Y-chromosome fertility genes. Chromosoma 1981, 83, 593–617.
- Kennison, J.A. The genetic and cytological organization of the Y chromosome of Drosophila melanogaster. Genetics 1981, 98, 529–548.
- Hazelrigg, T.; Fornili, P.; Kaufman, T.C. A cytogenetic analysis of X-ray induced male steriles on the Y chromosome of Drosophila melanogaster. Chromosoma 1982, 87, 535–559.
- Gatti, M.; Pimpinelli, S. Functional elements in Drosophila melanogaster heterochromatin. Annu. Rev. Genet. 1992, 26, 239–275.
- Zhang, P.; Stankiewicz, R.L. Y-linked male sterile mutations induced by P element in Drosophila melanogaster. Genetics 1998, 150, 735–744.
- Gatti, M.; Pimpinelli, S. Cytological and genetic analysis of the Y chromosome of Drosophila melanogaster. Chromosoma 1983, 88, 349–373.
- Zhang, J.; Luo, J.; Chen, J.; Dai, J.; Montell, C. The Role of Y Chromosome Genes in Male Fertility in Drosophila melanogaster. Genetics 2020, 215, 623–633.
- Carvalho, A.B.; Lazzaro, B.P.; Clark, A.G. Y chromosomal fertility factors kl-2 and kl-3 of Drosophila melanogaster encode dynein heavy chain polypeptides. Proc. Natl. Acad. Sci. USA 2000, 97, 13239–13244.
- Goldstein, L.S.B.; Hardy, R.W.; Lindsley, D.L. Structural genes on the Y chromosome of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1982, 79, 7405–7409.
- Gepner, J.; Hays, T.S. A fertility region on the Y chromosome of Drosophila melanogaster encodes a dynein microtubule motor. Proc. Natl. Acad. Sci. USA 1993, 90, 11132–11136.
- Rasmusson, K.; Serr, M.; Gepner, J.; Gibbons, I.; Hays, T.S. A family of dynein genes in Drosophila melanogaster. Mol. Biol. Cell 1994, 5, 45–55.
- Ishikawa, T. Axoneme Structure from Motile Cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028076.
- Yu, Z.; Ren, M.; Wang, Z.; Zhang, B.; Rong, Y.S.; Jiao, R.; Gao, G. Highly efficient genome modifications mediated by CRISPR/Cas9 in Drosophila. Genetics 2013, 195, 289–291.
- Hafezi, Y.S.S.; Tarrash, S.R.; Wolfner, M.F.; Clark, A.G. Dissecting fertility functions of Drosophila Y chromosome genes with CRISPR. Genetics 2020, 214, 977–990.
- Mahadevaraju, S.; Fear, J.M.; Akeju, M.; Galletta, B.J.; Pinheiro, M.M.L.S.; Avelino, C.C.; Cabral-de-Mello, D.C.; Conlon, K.; Dell’Orso, S.; Demere, Z.; et al. Dynamic sex chromosome expression in Drosophila male germ cells. Nat. Commun. 2021, 12, 892.
- Fingerhut, J.M.; Moran, J.V.; Yamashita, Y.M. Satellite DNA-containing gigantic introns in a unique gene expression program during Drosophila spermatogenesis. PLoS Genet. 2019, 15, e1008028.
- Fingerhut, J.M.; Yamashita, Y.M. mRNA localization mediates maturation of cytoplasmic cilia in Drosophila spermatogenesis. J. Cell Biol. 2020, 219, e202003084.
- Bonaccorsi, S.; Pisano, C.; Puoti, F.; Gatti, M. Y chromosome loops in Drosophila melanogaster. Genetics 1988, 120, 1015–1034.
- Bonaccorsi, S.; Gatti, M.; Pisano, C.; Lohe, A. Transcription of a satellite DNA on two Y chromosome loops of Drosophila melanogaster. Chromosoma 1990, 99, 260–266.
- Piergentili, R.; Mencarelli, C. Drosophila melanogaster kl-3 and kl-5 Y-loops harbor triple-stranded nucleic acids. J. Cell Sci. 2008, 121, 1605–1612.
- Redhouse, J.L.; Mozziconacci, J.; White, R.A. Co-transcriptional architecture in a Y loop in Drosophila melanogaster. Chromosoma 2011, 120, 399–407.
- Liao, S.E.; Ai, Y.; Fukunaga, R. An RNA-binding protein Blanks plays important roles in defining small RNA and mRNA profiles in Drosophila testes. Heliyon 2018, 4, e00706.
- Zhu, L.; Fukunaga, R. RNA-binding protein Maca is crucial for gigantic male fertility factor gene expression, spermatogenesis, and male fertility, in Drosophila. PLoS Genet. 2021, 17, e1009655.
- Hundertmark, T.; Kreutz, S.; Merle, N.; Nist, A.; Lamp, B.; Stiewe, T.; Brehm, A.; Renkawitz-Pohl, R.; Rathke, C. Drosophila melanogaster tPlus3a and tPlus3b ensure full male fertility by regulating transcription of Y-chromosomal, seminal fluid, and heat shock genes. PLoS ONE 2019, 14, e0213177.
- Cheng, M.H.; Maines, J.Z.; Wasserman, S.A. Biphasic subcellular localization of the DAZL-related protein boule in Drosophila spermatogenesis. Dev. Biol. 1998, 204, 567–576.
- Heatwole, V.M.; Haynes, S.R. Association of RB97D, an RRM protein required for male fertility, with a Y chromosome lampbrush loop in Drosophila spermatocytes. Chromosoma 1996, 105, 285–292.
- Ceprani, F.; Raffa, G.D.; Petrucci, R.; Piergentili, R. Autosomal mutations affecting Y chromosome loops in Drosophila melanogaster. BMC Genet. 2008, 9, 32.
- Hackstein, J.H.; Leoncini, O.; Beck, H.; Peelen, G.; Hennig, W. Genetic fine structure of the Y chromosome of Drosophila hydei. Genetics 1982, 101, 257–277.
- Vogt, P.; Hennig, W. Molecular structure of the lampbrush loops nooses of the Y chromosome of Drosophila hydei: I. The Y chromosome-specific repetitive DNA sequence family ay1 is dispersed in the loop DNA. Chromosoma 1986, 94, 449–458.
- Vogt, P.; Hennig, W. Molecular structure of the lampbrush loops nooses of the Y chromosome of Drosophila hydei: II. DNA sequences with homologies to multiple gemonic locations are major constituents of the loop. Chromosoma 1986, 94, 459–467.
- Wlaschek, M.; Awgulewitsch, A.; Bunemann, H. Structure and function of Y chromosomal DNA. I. Sequence organization and localization of four families of repetitive DNA on the Y chromosome of Drosophila hydei. Chromosoma 1988, 96, 145–158.
- Piergentili, R. Evolutionary conservation of lampbrush-like loops in drosophilids. BMC Cell Biol. 2007, 8, 35.
- Huijser, P.; Beckers, L.; Top, B.; Hermans, M.; Sinke, R.; Hennig, W. Poly·poly is highly transcribed in the testes of Drosophila hydei. Chromosoma 1990, 100, 48–55.
- Trapitz, P.; Glatzer, K.H.; Bunemann, H. Towards a physical map of the fertility genes on the heterochromatic Y chromosome of Drosophila hydei: Families of repetitive sequences transcribed on the lampbrush loops Nooses and Threads are organized in extended clusters of several hundred kilobases. Mol. Gen. Genet. 1992, 235, 221–234.
- Hochstenbach, R.; Brand, R.; Hennig, W. Transcription of repetitive DNA sequences in the lampbrush loop pair Nooses formed by sterile alleles of fertility gene Q on the Y chromosome of Drosophila hydei. Mol. Gen. Genet. 1994, 244, 653–660.
- Shermoen, A.W.; O’Farrell, P.H. Progression of the cell cycle through mitosis leads to abortion of nascent transcripts. Cell 1991, 67, 303–310.
- Koenig, M.; Beggs, A.H.; Moyer, M.; Scherpf, S.; Heindrich, K.; Bettecken, T.; Meng, G.; Müller, C.R.; Lindlöf, M.; Kaariainen, H.; et al. The molecular basis for Duchenne versus Becker muscular dystrophy: Correlation of severity with type of deletion. Am. J. Hum. Genet. 1989, 45, 498–506.
- Pozzoli, U.; Sironi, M.; Cagliani, R.; Comi, G.P.; Bardoni, A.; Bresolin, N. Comparative analysis of the human dystrophin and utrophin gene structures. Genetics 2002, 160, 793–798.
- Le Rumeur, E. Dystrophin and the two related genetic diseases, Duchenne and Becker muscular dystrophies. Bosn. J. Basic Med. Sci. 2015, 15, 14–20.
- Gao, Q.Q.; McNally, E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol. 2015, 5, 1223–1239.
- Verhaart, I.E.C.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386.
- Pastinen, T. Genome-wide allele-specific analysis: Insights into regulatory variation. Nat. Rev. Genet. 2010, 11, 533–538.
- Gaur, U.; Li, K.; Mei, S.; Liu, G. Research progress in allele-specific expression and its regulatory mechanisms. J. Appl. Genet. 2013, 54, 271–283.
- McStay, B. Nucleolar dominance: A model for rRNA gene silencing. Genes Dev. 2006, 20, 1207–1214.
- Bayes, J.J.; Malik, H.S. Altered heterochromatin binding by a hybrid sterility protein in Drosophila sibling species. Science 2009, 326, 1538–1541.
- Araripe, L.O.; Tao, Y.; Lemos, B. Interspecific Y chromosome variation is sufficient to rescue hybrid male sterility and is influenced by the grandparental origin of the chromosomes. Heredity 2016, 116, 516–522.
- Durica, D.S.; Krider, H.M. Studies on the ribosomal RNA cistrons in interspecific Drosophila hybrids: I. Nucleolar dominance. Dev. Biol. 1977, 59, 62–74.
- Lewis, M.S.; Cheverud, J.M.; Pikaard, C.S. Evidence for nucleolus organizer regions as the units of regulation in nucleolar dominance in Arabidopsis thaliana interecotype hybrids. Genetics 2004, 167, 931–939.
- Greil, F.; Ahmad, K. Nucleolar dominance of the Y chromosome in Drosophila melanogaster. Genetics 2012, 191, 1119–1128.
- Zhou, J.; Sackton, T.B.; Martinsen, L.; Lemos, B.; Eickbush, T.H.; Hartl, D.L. Y chromosome mediates ribosomal DNA silencing and modulates the chromatin state in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 9941–9946.
- Preuss, S.; Pikaard, C.S. rRNA gene silencing and nucleolar dominance: Insights into a chromosome-scale epigenetic on/off switch. Biochim. Biophys. Acta. 2007, 1769, 383–392.
- Tucker, S.; Vitins, A.; Pikaard, C.S. Nucleolar dominance and ribosomal RNA gene silencing. Curr. Opin. Cell Biol. 2010, 22, 351–356.
- Moss, T.; Stefanovsky, V.Y. At the center of eukaryotic life. Cell 2002, 109, 545–548.
- Long, E.O.; Dawid, I.B. Repeated genes in eukaryotes. Annu. Rev. Biochem. 1980, 49, 727–764.
- Sinclair, D.A.; Guarente, L. Extrachromosomal rDNA circles—A cause of aging in yeast. Cell 1997, 91, 1033–1042.
- Helmrich, A.; Ballarino, M.; Nudler, E.; Tora, L. Transcription-replication encounters, consequences and genomic instability. Nat. Struct. Mol. Biol. 2013, 20, 412–418.
- Lyckegaard, E.M.; Clark, A.G. Evolution of ribosomal RNA gene copy number on the sex chromosomes of Drosophila melanogaster. Mol. Biol. Evol. 1991, 8, 458–474.
- Gibbons, J.G.; Branco, A.T.; Godinho, S.A.; Yu, S.; Lemos, B. Concerted copy number variation balances ribosomal DNA dosage in human and mouse genomes. Proc. Natl. Acad. Sci. USA 2015, 112, 2485–2490.
- Ganley, A.R.; Kobayashi, T. Ribosomal DNA and cellular senescence: New evidence supporting the connection between rDNA and aging. FEMS Yeast Res. 2014, 14, 49–59.
- Kobayashi, T. How does genome instability affect lifespan?: Roles of rDNA and telomeres. Genes Cells 2011, 16, 617–624.
- Eickbush, T.H.; Burke, W.D.; Eickbush, D.G.; Lathe, W.C., III. Evolution of R1 and R2 in the rDNA units of the genus Drosophila. Genetica 1997, 100, 49–61.
- Hawley, R.S.; Marcus, C.H. Recombinational controls of rDNA redundancy in Drosophila. Annu. Rev. Genet. 1989, 23, 87–120.
- Peng, J.C.; Karpen, G.H. H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat. Cell Biol. 2007, 9, 25–35.
- Warsinger-Pepe, N.; Li, D.; Yamashita, Y.M. Regulation of Nucleolar Dominance in Drosophila melanogaster. Genetics 2020, 214, 991–1004.
- Lu, K.L.; Nelson, J.O.; Watase, G.J.; Warsinger-Pepe, N.; Yamashita, Y.M. Transgenerational dynamics of rDNA copy number in Drosophila male germline stem cells. eLife 2018, 7, e32421.
- McKee, B.D.; Karpen, G.H. Drosophila ribosomal RNA genes function as an X-Y pairing site during male meiosis. Cell 1990, 61, 61–72.
- Watase, G.J.; Yamashita, Y.M. Non-random sister chromatid segregation mediates rDNA copy number maintenance in Drosophila. bioRxiv 2022.
- Yadlapalli, S.; Yamashita, Y.M. DNA asymmetry in stem cells—Immortal or mortal? J. Cell Sci. 2013, 126, 4069–4076.
- Roussel, P.; André, C.; Comai, L.; Hernandez-Verdun, D. The rDNA transcription machinery is assembled during mitosis in active NORs and absent in inactive NORs. J. Cell Biol. 1996, 133, 235–246.
- Héliot, L.; Mongelard, F.; Klein, C.; O’Donohue, M.F.; Chassery, J.M.; Robert-Nicoud, M.; Usson, Y. Nonrandom distribution of metaphase AgNOR staining patterns on human acrocentric chromosomes. J. Histochem. Cytochem. 2000, 48, 13–20.
- Hori, Y.; Shimamoto, A.; Kobayashi, T. The human ribosomal DNA array is composed of highly homogenized tandem clusters. Genome Res. 2021, 31, 1971–1982.
- Le Thomas, A.; Stuwe, E.; Li, S.; Du, J.; Marinov, G.; Rozhkov, N.; Chen, Y.C.; Luo, Y.; Sachidanandam, R.; Toth, K.F.; et al. Transgenerationally inherited piRNAs trigger piRNA biogenesis by changing the chromatin of piRNA clusters and inducing precursor processing. Genes Dev. 2014, 28, 1667–1680.
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 2019, 20, 89–108.
- Ramat, A.; Simonelig, M. Functions of PIWI Proteins in Gene Regulation: New Arrows Added to the piRNA Quiver. Trends Genet. 2021, 37, 188–200.
- Aravin, A.A.; Hannon, G.J.; Brennecke, J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science 2007, 318, 761–764.
- Malone, C.D.; Brennecke, J.; Dus, M.; Stark, A.; McCombie, W.R.; Sachidanandam, R.; Hannon, G.J. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell 2009, 137, 522–535.
- Gainetdinov, I.; Colpan, C.; Arif, A.; Cecchini, K.; Zamore, P.D. A Single Mechanism of Biogenesis, Initiated and Directed by PIWI Proteins, Explains piRNA Production in Most Animals. Mol. Cell 2018, 71, 775–790.e5.
- Brennecke, J.; Aravin, A.A.; Stark, A.; Dus, M.; Kellis, M.; Sachidanandam, R.; Hannon, G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 2007, 128, 1089–1103.
- Mohn, F.; Sienski, G.; Handler, D.; Brennecke, J. The Rhino-Deadlock-Cutoff Complex Licenses Noncanonical Transcription of Dual-Strand piRNA Clusters in Drosophila. Cell 2014, 157, 1364–1379.
- Chen, Y.A.; Stuwe, E.; Luo, Y.; Ninova, M.; Le Thomas, A.; Rozhavskaya, E.; Li, S.; Vempati, S.; Laver, J.D.; Patel, D.J.; et al. Cutoff Suppresses RNA Polymerase II Termination to Ensure Expression of piRNA Precursors. Mol. Cell. 2016, 63, 97–109.
- Zhang, Z.; Wang, J.; Schultz, N.; Zhang, F.; Parhad, S.S.; Tu, S.; Vreven, T.; Zamore, P.D.; Weng, Z.; Theurkauf, W.E. The HP1 homolog rhino anchors a nuclear complex that suppresses piRNA precursor splicing. Cell 2014, 157, 1353–1363.
- Kotov, A.A.; Adashev, V.E.; Godneeva, B.K.; Ninova, M.; Shatskikh, A.S.; Bazylev, S.S.; Aravin, A.A.; Olenina, L.V. piRNA silencing contributes to interspecies hybrid sterility and reproductive isolation in Drosophila melanogaster. Nucleic Acids Res. 2019, 47, 4255–4271.
- Aravin, A.A.; Naumova, N.M.; Tulin, A.V.; Vagin, V.V.; Rozovsky, Y.M.; Gvozdev, V.A. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 2001, 11, 1017–1027.
- Vagin, V.V.; Sigova, A.; Li, C.; Seitz, H.; Gvozdev, V.; Zamore, P.D. A distinct small RNA pathway silences selfish genetic elements in the germline. Science 2006, 313, 320–324.
- Balakireva, M.D.; Shevelyov, Y.Y.; Nurminsky, D.I.; Livak, K.J.; Gvozdev, V.A. Structural organization and diversification of Y-linked sequences comprising Su(Ste) genes in Drosophila melanogaster. Nucleic Acids Res. 1992, 20, 3731–3736.
- Adashev, V.E.; Kotov, A.A.; Bazylev, S.S.; Shatskikh, A.S.; Aravin, A.A.; Olenina, L.V. Stellate Genes and the piRNA Pathway in Speciation and Reproductive Isolation of Drosophila melanogaster. Front. Genet. 2021, 11, 610665.
- Hurst, L.D. Is Stellate a relict meiotic driver? Genetics 1992, 130, 229–230.
- Chen, P.; Luo, Y.; Aravin, A.A. RDC complex executes a dynamic piRNA program during Drosophila spermatogenesis to safeguard male fertility. PLoS Genet. 2021, 17, e1009591.
- Olenkina, O.M.; Egorova, K.S.; Kibanov, M.V.; Gervaziev, Y.V.; Gvozdev, V.A.; Olenina, L.V. Promoter contribution to the testis-specific expression of Stellate gene family in Drosophila melanogaster. Gene 2012, 499, 143–153.
- Ishizu, H.; Iwasaki, Y.W.; Hirakata, S.; Ozaki, H.; Iwasaki, W.; Siomi, H.; Siomi, M.C. Somatic Primary piRNA Biogenesis Driven by cis-Acting RNA Elements and trans-Acting Yb. Cell Rep. 2015, 12, 429–440.
- Reddy, H.M.; Bhattacharya, R.; Tiwari, S.; Mishra, K.; Annapurna, P.; Jehan, Z.; Praveena, N.M.; Alex, J.L.; Dhople, V.M.; Singh, L.; et al. Y chromosomal noncoding RNAs regulate autosomal gene expression via piRNAs in mouse testis. BMC Biol. 2021, 19, 198.
- Kiuchi, T.; Koga, H.; Kawamoto, M.; Shoji, K.; Sakai, H.; Arai, Y.; Ishihara, G.; Kawaoka, S.; Sugano, S.; Shimada, T.; et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 2014, 509, 633–636.
- Ellison, C.; Bachtrog, D. Recurrent gene co-amplification on Drosophila X and Y chromosomes. PLoS Genet. 2019, 15, e1008251.
- Sarkar, A.; Maji, R.K.; Saha, S.; Ghosh, Z. piRNAQuest: Searching the piRNAome for silencers. BMC Genom. 2014, 15, 555.
- Özata, D.M.; Yu, T.; Mou, H.; Gainetdinov, I.; Colpan, C.; Cecchini, K.; Kaymaz, Y.; Wu, P.H.; Fan, K.; Kucukural, A.; et al. Evolutionarily conserved pachytene piRNA loci are highly divergent among modern humans. Nat. Ecol. Evol. 2020, 4, 156–168.
- Ha, H.; Song, J.; Wang, S.; Kapusta, A.; Feschotte, C.; Chen, K.C.; Xing, J. A comprehensive analysis of piRNAs from adult human testis and their relationship with genes and mobile elements. BMC Genom. 2014, 15, 545.
- Chalvet, F.; di Franco, C.; Terrinoni, A.; Pelisson, A.; Junakovic, N.; Bucheton, A. Potentially active copies of the gypsy retroelement are confined to the Y chromosome of some strains of Drosophila melanogaster possibly as the result of the female-specific effect of the flamenco gene. J. Mol. Evol. 1998, 46, 437–441.
- Gvozdev, V.A.; Kogan, G.L.; Usakin, L.A. The Y chromosome as a target for acquired and amplified genetic material in evolution. Bioessays 2005, 27, 1256–1262.
- Chang, C.H.; Chavan, A.; Palladino, J.; Wei, X.; Martins, N.M.C.; Santinello, B.; Chen, C.C.; Erceg, J.; Beliveau, B.J.; Wu, C.T.; et al. Islands of retroelements are major components of Drosophila centromeres. PLoS Biol. 2019, 17, e3000241.
- Berloco, M.; Fanti, L.; Sheen, F.; Levis, R.W.; Pimpinelli, S. Heterochromatic distribution of HeT-A- and TART-like sequences in several Drosophila species. Cytogenet. Genome Res. 2005, 110, 124–133.
- Cacchione, S.; Cenci, G.; Raffa, G.D. Silence at the End: How Drosophila Regulates Expression and Transposition of Telomeric Retroelements. J. Mol. Biol. 2020, 432, 4305–4321.
- Lawlor, M.A.; Cao, W.; Ellison, C.E. A transposon expression burst accompanies the activation of Y-chromosome fertility genes during Drosophila spermatogenesis. Nat. Commun. 2021, 12, 6854.
- Cox, D.N.; Chao, A.; Lin, H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 2000, 127, 503–514.
- Kibanov, M.V.; Kotov, A.A.; Olenina, L.V. Multicolor fluorescence imaging of whole-mount Drosophila testes for studying spermatogenesis. Anal. Biochem. 2013, 436, 55–64.
