Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Food Science & Technology
Aflatoxins are the mycotoxins of the greatest concern to food safety due to their wide distribution in foods and feeds and their high toxicities. Since their discovery, aflatoxins have been associated with liver cancer, with peanut, maize and their derivatives being the main vehicles. Geographically, tropical and subtropical regions are the most affected by aflatoxins as food and feed contaminants and as chemical hazards that contribute greatly to the high incidence of a number of devastating chronic diseases and aflatoxicosis outbreaks.
- aflatoxins
- tumorigenicity
- carcinogenicity
- acute toxicity
1. Chronic Diseases Caused by Aflatoxins
Repeated exposure to low doses of aflatoxins over a lifetime causes chronic diseases, the most frequent and severe of which is cancer. Although dietary intake of aflatoxins has been classically associated with primary liver cancer, i.e., HCC and bile duct hyperplasia [3], other organs, such as the kidney, the pancreas, the bladder, bone, viscera, etc., have also been reported to develop cancer upon exposure to these mycotoxins [1]. In addition, lung [4] and skin [5] occupational cancers were also associated with aflatoxins via inhalation and direct contact, respectively. Chronic exposure to aflatoxins causes a range of other severe diseases, including immunosuppression, teratogenicity, mutagenicity, cytotoxicity, and estrogenic effects in mammalians [6]. Moreover, aflatoxins are believed to be involved in nutritional disorders in children, such as kwashiorkor and growth faltering, probably by interfering with the absorption of micronutrients (e.g., zinc, iron, and vitamins), protein synthesis, and metabolic enzyme activities [2,7]. In domestic animals, feeds contaminated with sub-lethal doses of aflatoxins induce impaired productivity and reproduction, increased susceptibility to diseases, and reduced quality of the foods they produce [8]. Despite the insidious character of chronic aflatoxin-induced diseases, their impact on public health globally is more severe and more costly than acute aflatoxicosis. Although, the aflatoxicosis outbreaks induce hundreds of deaths at once in an intermittent manner, they can be prevented or interrupted upon analysis of suspect crops/foods, e.g., evident mould growth, and their disposal if aflatoxin levels exceed the regulatory standards.
Liver cancer is one of the most common and deadly type of cancer diseases whose occurrence has been strongly correlated with dietary exposure to aflatoxins, which is enhanced in the presence of other risk factors [2]. Notably, chronic infections with hepatitis virus B (HB) were shown to cause an increase in the potency of AFB1 by up to 60 times [9]. According to the most recent statistics given by the global cancer observatory of the IARC (http://gco.iarc.fr, accessed on 1 September 2019), 841,080 new cases of liver cancer causing 781,631 deaths were recorded globally in 2018. This corresponds to an age-standardized incidence rate of 9.3 per 100,000 and mortality rate of 93% ranking as the fifth cancer type and the first cause of cancer-induced mortality. Africa and Asia continue to be the leading continents in terms of new cases recorded each year, with 64,779 (7.7%) and 609,596 (72%) cases respectively, together representing about 80% of the total cases in the world. Aflatoxin B1 alone was estimated to cause 25,200 to 155,000 cases each year [10,11], 40% of which occur in sub-Saharan Africa only [2] where aflatoxin-induced liver cancer accounts for one-third of all liver cancer cases recorded in the whole African continent [12]. At the country level, China has the highest incidence of liver cancer in the world, with the vast majority being recorded in the Southern part of the country where the two main synergistic causative agents, exposure to dietary aflatoxins and HB chronic infections, are endemic and highest [3].
2. Mechanisms of Toxicity at Glance:
Aflatoxins exert various toxicological effects with different mechanisms, most of which are not yet fully elucidated. Intensive research has been carried out to investigate the mechanisms of the toxicity of aflatoxins to provide a scientific basis for the design of preventive and control means. Advanced knowledge in the field can also serve as a scientific tool for the provision of regulatory purposes by food safety authorities. The mutagenic effects of AFB1 have been the focus of most studies since the discovery of this aflatoxin and were ascribed mainly to the intermediate metabolite AFB1-exo-8,9 epoxide (AFBO) [13]. As a highly unstable molecule, AFBO reacts with cellular macromolecules, including nucleic acids, proteins, and phospholipids, to induce various genetic, metabolic, signalling, and cell structure disruptions [14,15,16]. However, increased evidence is being built up demonstrating equally dramatic or higher effects of AFB1 on cell function and integrity through the induction of oxidative stress (OS) [17,18,19]. Figure 1 summarizes the different toxicity mechanisms of AFB1 involving AFBO and OS to cause genotoxicity, immunotoxicity and acute intoxication by acting on genomic DNA, other functional macromolecules and immunocompetent cells.
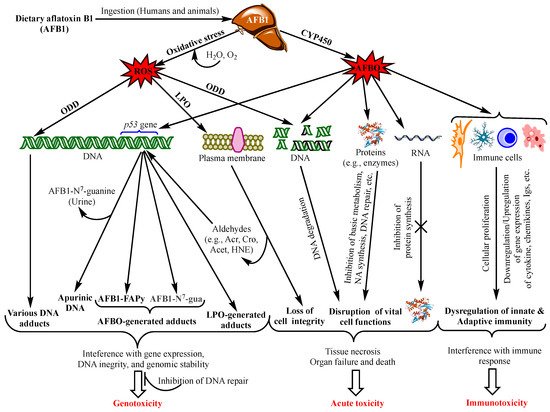
Figure 1. Main aflatoxin B1 toxicity mechanisms mediated by the oxidative stress and AFB1-exo-8,9 epoxide (AFBO; see text for explanations). NB: ROS also affect proteins, RNA molecules, and immunity as does AFBO (not shown in the figure. For details, see [20]). Abbreviations: AFBO: Aflatoxin B1-exo-8,9-epoxide; NA: Nucleic Acids; ROS: Reactive Oxygen Species; LPO: Lipid Peroxidation; ODD: Oxidative DNA Damage; Acr: Acrolein; Cro: Crotonaldehyde; Acet: Acetaldehyde; HNE: 4-Hydroxy-2-Nonenal; uFA: Unsaturated Fatty Acids; IL1β: Interleukin 1β, IL6: Interleukin 6; TNFα: Tumour Necrotizing Factor α; P-dG: Cyclic Propano-Deoxyguanosine; Igs: Immunoglobulins. See text for the other abbreviations.
3. Immunotoxicity
Increased frequency and severity, and prolonged healing of infectious diseases, in addition to decreased vaccination efficacies provided evidence that aflatoxins disrupt both innate and acquired/adaptive immunity [103,104,105,106,107]. The general mechanisms of AFB1 immunotoxicity via AFBO is presented in Figure 1. It can be seen from this figure that AFBO interacts with immunocompetent cells throughout the body to affect their proliferation and/or production of immune response mediators, thereby disrupting the innate and adaptive immunity. Although most studies to illustrate these mechanisms have been carried out on animals, the immunotoxicity of AFB1 has also been substantiated in vitro on human cell lines and in case–control studies in highly exposed regions, e.g., Ghana [105,108,109,110]. However, few studies to our knowledge have investigated the immunotoxicity of aflatoxins other than AFB1 or its combination with other mycotoxins [111,112,113,114]. Meanwhile, there has been a general agreement that low or moderate concentrations of AFB1 have no or a marginal immunotoxicity, and that cell-mediated immunity (CMI) is more susceptible to aflatoxins than humoral immunity [105,112,115,116].
Regarding the mode of immunomodulation of the immune function by aflatoxins, most of the available data suggest that they mainly exert suppressive effects; however, in vitro and in vivo studies have demonstrated that they can also dysregulate the immune response via immunostimulatory effects [122,123].
4. Teratogenicity
The exposure of pregnant females or birds to aflatoxins can affect embryos in utero or in fertilized eggs, respectively, producing various adverse health effects and different pathological gestation/incubation outcomes [167]. In mammalians, systemic blood circulation in highly exposed mothers conveys aflatoxins or their toxic metabolites to foetuses, as has been substantiated in highly exposed pregnant women from African and Asian countries, as well as in animals. Indeed, aflatoxins and/or biomarkers derived thereof, e.g., aflatoxin metabolites, and aflatoxin-DNA and aflatoxin–albumin adducts, were detected in the cord blood of the foetus or in both foetal cord and maternal blood samples [168,169,170,171,172]. Accordingly, it was concluded that aflatoxins or their metabolites in pregnant women are transmitted to the foetus and metabolized through the same pathways as in adults [172]. Therefore, the pregnancy of highly exposed mothers is prone to various outcomes, including foetal growth restriction, foetal loss, and premature birth. Growth restriction has been documented in humans and animals where an inverse relationship between the birthweight and the amounts of appropriate biomarkers in the cord blood has been extensively demonstrated [169,173,174,175]. Conversely, few studies have related high-aflatoxin exposure of pregnant women to stillbirth, while studies on the association of high aflatoxin intake by pregnant women with premature birth and foetal loss are either non conclusive [175] or lacking [174]. On the contrary, decrease in live birth and litter size, impairment of organ development, and skeletal anomalies in offspring have been demonstrated in animals given aflatoxins at daily doses ranging between 0 (nil) and 100 µg/kg bw, which was explained by the binding of aflatoxins to the DNA and the hindrance of protein synthesis [167,176,177,178,179,180]. This view can be applied to humans, as aflatoxins bind to human DNA in the same way, but it remains to be clinically demonstrated.
In addition to the above-mentioned adverse health effects, an aflatoxin-rich diet in pregnant females affects their health and expose their foetuses to indirect consequences with congenital abnormalities. For example, upregulation of maternal pro-inflammatory cytokines and/or downregulation of anti-inflammatory cytokines induce systemic inflammation that impairs the placental growth and causes its insufficiency ultimately leading to poor foetal growth, miscarriage and stillbirth, or prematurity [113,168,173]. Furthermore, the cytotoxic activity of aflatoxins induces anaemia in mothers by lysing red blood cells or interfering with nutrients, e.g., iron, selenium, and vitamins, absorption with consequent poor foetal growth and/or prematurity [181,182,183]. The association of anaemia and high aflatoxin intake, as determined by AFB-albumin adduct in the mothers’ serum, was demonstrated in a cross-sectional study on Ghanaian women [175]. On the other hand, the association of anaemia to red blood cell lysis by aflatoxins was demonstrated in vitro and in animal species dosed with 0.5 to 1.0 mg/kg bw [184,185,186,187]. However, it appears that the environmentally relevant levels of aflatoxins remain below the doses that can elicit red blood cell lysis in humans. Conversely, there is a lack of evidence on the association between inflammation-induced anaemia in pregnant women and their exposure to aflatoxins. As matter of fact, there are many gaps in the knowledge of doses, mechanisms, and outcomes of exposure to aflatoxins in pregnant women that require more attention and rigorous scientific approaches to be clearly understood and eventually avoided to ensure safe pregnancy and birth.
5. Other Adverse Health Effects of Chronic Exposure to Aflatoxins
In addition to the major toxicological effects reviewed above, aflatoxins exert various other adverse health conditions with overlapping mechanisms and risk factors. These include malnutrition diseases (faltering and stunting), retarded physical and mental maturity, reproduction and sexuality issues OK, and nervous system diseases (neurodegenerative diseases and neuroblastoma) [2,188,189,190]. However, most of the latter effects have been scarcely investigated to cover the main pertaining aspects from applied and mechanistic standpoints. Therefore, further studies are needed for clearer insights on these issues to have an accurate and realistic opinion on the risk they may pose to the public health. This section addresses malnutrition and neurodegenerative diseases, which have been relatively well studied.
5.1. Aflatoxins and Malnutrition
Malnutrition is probably one of the above-mentioned aspects that has received the most attention due to its impact on childhood in many developing countries, where children are already facing food shortages. It is essential to ensure children receive balanced and nutrition for healthy growth, and hence be well prepared to adulthood as active and productive individuals. Exposure to aflatoxins exacerbates such poor nutritional status by interfering with the absorption of vitamins and minerals, as has been shown for vitamins A, C, and E, and selenium [191]. This not only deprives children/consumers from these essential micronutrients, but also increases their susceptibility to aflatoxins that they normally detoxify owing to their inherent antioxidant or CYPP450 inhibitory activities [101,167,192]. As a result, exposed children may experience growth disorders from the gestational stage as discussed above, throughout adulthood, with stunted and retarded physical and mental maturity [193]. Indeed, in African countries, growth faltering among children below 5 years old was correlated with chronic exposure to high levels of aflatoxins when they rely on local agricultural products, e.g., maize, peanut, and derivatives as staple foods [194]. On the other hand, severe protein energy malnutrition (PEM) diseases, such as Kwashiorkor and marasmic kwashiorkor, have been associated with chronic exposure to high levels of dietary aflatoxins in different African countries [195,196,197,198]. However, since all the relevant studies were conducted in poor household environments where children were invariably fed on local agricultural products with poor nutritional and hygienic quality and limited availability, PEM could be due to the limited access to enough nutritious foods, rather than to aflatoxin intake. To address this particular issue, a study has been conducted on malnourished Soudanese children with Kwashiorkor, marasmic kwashiorkor, or marasmus. The results of the study revealed that a group of kwashiorkor and marasmic kwashiorkor children had significantly higher levels of AFB1 and its derivative aflatoxicol in their sera and urine compared with a group of malnourished children with marasmus and a group of age-matched normally nourished children [197]. Accordingly, the authors concluded that kwashiorkor is definitely correlated with high chronic exposure to aflatoxins as either secondary to liver damage or an aetiological factor of the disease, which remains to be further substantiated by appropriately designed future studies [197].
5.2. Aflatoxins and Neurodegenerative Diseases
In addition to the classically known adverse health effects of aflatoxins, there is increasing body of evidence that chronic exposure to aflatoxins can also be responsible for neurodegenerative disorders. The AFBO and ROS generated by CYP450 enzymes and aflatoxin-induced oxidative stress, respectively, react with functional macromolecules in neuronal brain cells where they inhibit lipid and protein synthesis to induce their degeneration [199]. Aflatoxins were also reported to disrupt the structure and function of mitochondria of brain cells, which impedes oxidative phosphorylation and leads to their apoptosis [200]. In addition, the detection of aflatoxins in brain tissues of kwashiorkor-deceased children and their association with Rey’s disease (cerebral edema and neuronal degeneration) is a strong indication that aflatoxins can cross the brain-blood barrier and infiltrate the nervous system that they degenerate [21,188]. Although scarce, epidemiological studies have demonstrated the neurotoxicity of aflatoxins in humans and animals. In a recent study, rats dosed with 1/600th their LD50 dysregulated the levels of biochemical biomarkers of the oxidative stress indicative of neurodegenerative disorders, which were corroborated by histopathological and immunohistochemical tests showing vasodilation, necrosis and astrocytes gliosis [188]. In addition to the oxidative stress, aflatoxins induce neurodegenerative disorders by dysregulating the immune response of immunocompetent cells and creating proinflammatory conditions in the central nervous system [21].
6. Acute Toxicity
The mechanism of acute aflatoxicosis is poorly understood, although many authors refer to the interaction between aflatoxins and macromolecules (proteins, phospholipids, and nucleic acids) with a consequent formation of various adducts, which in turn interferes with the physiological and structural functions of the macromolecules. In particular, aflatoxin-protein adducts have been the most frequently associated with acute intoxication, as this blocks protein synthesis, especially the enzymes involved in vital functions, such metabolic pathways, protein synthesis, DNA replication and repair, and immune response (Figure 1). Additionally, there is increasing evidence that aflatoxin-phospholipid adducts and ROS-induced LPO are the main reasons for the disruption of the integrity and function of the membranes of the cells, mitochondria, and endoplasmic reticulum [14,20]. Moreover, severe DNA fragmentation upon exposure to high doses of aflatoxins is another major effect of acute aflatoxicosis (Figure 1), as was observed in the testicular tissues of mice injected with a daily dose of 20 µg AFB1/kg bw for 21 days [62]. However, a recent study on the acute toxicity of AFB1 in poultry suggested that aflatoxin–dihydrodiol (AF–dhd) is the main metabolite responsible for acute aflatoxicosis for being the pivotal metabolite leading to the formation of aflatoxin–albumin adducts [117]. According to the authors, AF–dhd derives from aflatoxin-exo 8,9-epoxide and forms the aflatoxin–albumin adducts via aflatoxin-aldehyde bypassing the formation of aflatoxin–dialcohol of the detoxification pathway [13]; and the more rapidly and abundantly AF–dhd is formed, the higher is the mortality rate. Moreover, the metabolism of AFB2a as a dietary contaminant or as an AFB1-phase I metabolite was also suggested to be involved in acute toxicity; apart from the formation of aflatoxin–albumin adducts, AFB2a was also reported to bind covalently with cellular proteins and phospholipids, yielding lipid- and protein-adducts, possibly leading to acute aflatoxicosis [14].
It should be pointed out, however, that chronic exposure to low doses of aflatoxins can produce similar effects as those observed in acute aflatoxicosis; however, their effects can be mitigated by detoxifying phase II enzymes and cellular antioxidant defense mechanisms, or by DNA repair to prevent mutations, as discussed above (Section 4.). Alternatively, these effects accumulate progressively with continuous exposure to low doses to, ultimately, evolve into liver cancer as the typical outcome of chronic exposure. Therefore, acute aflatoxicosis may result from an abrupt accentuation of most or all of the above-mentioned damages in a short time when the dose is too high. Although such a high dose remains to be specified, an overwhelming amount of aflatoxins can overcome the detoxifying capacity of the cell and drive the metabolism of the toxins towards the production of toxic metabolites causing severe DNA damage, the disruption of cell cycle progression, DNA fragmentation, metabolic disorders, cytotoxicity, and tissue necrosis, eventually leading to organ failure (Figure 1) in a short period. This may hold especially true as the adverse aflatoxin effects are cumulative [201,202]. For example, FAPy-DNA adduct burden that triggers tumorigenesis in rats was estimated to be one adduct per 250,000 nucleotides, i.e., 40,000 adducts/cell [203], which can either accumulate progressively with chronic exposure, or be reached in a short time in the case of exposure to abnormally high doses of AFB1.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph17020423
This entry is offline, you can click here to edit this entry!
