Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Nowadays ROD is a classic term that should be exclusively used to define histological bone lesions related to CKD; this spectrum of abnormalities could be assessed only by BB according to the TMV (turnover, mineralization, volume) classification system, recommended by KDIGO in 2006 to standardize the changes.
- CKD-MBD
- chronic kidney disease
- renal osteodystrophy
- osteoporosis
- bone biopsy
- fractures
1. Renal Osteodystrophy (ROD)
Definition. Nowadays ROD is a classic term that should be exclusively used to define histological bone lesions related to CKD [2]; this spectrum of abnormalities could be assessed only by BB according to the TMV (turnover, mineralization, volume) classification system, recommended by KDIGO in 2006 to standardize the changes [3,4].
TMV classification system. Bone turnover reflects the rate of skeletal remodeling, which is related to the process of bone resorption and formation. In CKD, it varies widely and can be low, normal, or high, and the extremes have been associated with vascular calcifications and higher mortality. Despite the identification of new hormones and markers of bone formation/resorption (e.g., bone alkaline phosphatase, tartrate-resistant acid phosphatase 5b, osteocalcin, sclerostin, klotho and fibroblast growth factor 23), diagnostic accuracy has been difficult to establish in CKD patients [4]. In contrast, turnover assessed with histomorphometry by significant increase or reduction in osteoblast and osteoclast numbers and by dynamic measurements of osteoblast function, using the tetracycline double-labeled technique, is accepted as a robust measurement [2,4].
Mineralization reflects the physiological share of collagen that becomes calcified during bone formation. In CKD, it can be normal or deficient. Routine serum biomarkers (e.g., calcium, phosphorus, and total alkaline phosphatase) are not specific or sensitive enough to identify mineralization disorders; it is assessed with histomorphometry both by static measurements of osteoid (volume, thickness) and by dynamic tetracycline-based measurements of mineralization lag time [2].
Volume reflects the amount of bone per unit of tissue. In CKD it can be low, normal, or high and various imaging tools could help (i.e., dual-energy X-ray absorptiometry, high resolution peripheral quantitative computerized tomography, or trabecular bone score software). In clinical practice, none of these methods allow evaluation of turnover and mineralization and there are few data on CKD patients. Volume is assessed with histomorphometry by static measurements [2].
Histological classification. The different combinations of each of these histological descriptors characterize specific entities in CKD. The classic description includes secondary hyperparathyroidism indicated as osteitis fibrosa, adynamic bone disease, osteomalacia, and mixed uremic osteodystrophy. The TMV classification system is shown in Table 1. This standardized nomenclature for patterns is needed to promote a more widespread understanding and to facilitate comparisons from various research reports [1,2,3]. Suggestive representations of these different forms of ROD are obtained by BB approach, allowing the study of each component of the bone multicellular unit (Figure 1) [5].
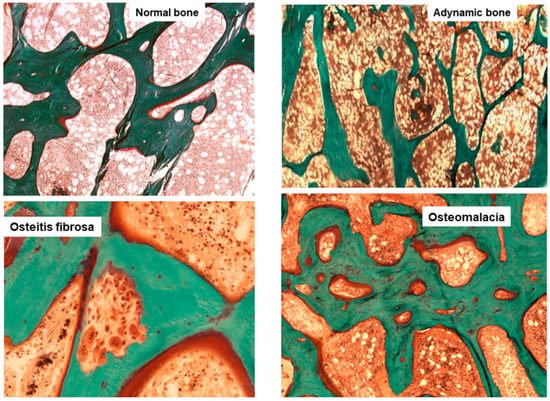
Figure 1. Main histomorphometric patterns of ROD in Goldner trichrome-stained bone section according to TMV classification system.
Table 1. Histologic classification of renal osteodystrophy in CKD with TMV classification.
| Type of Renal Osteodystrophy | Turnover | Mineralization | Volume |
|---|---|---|---|
| Osteomalacia | Low | Abnormal | Low to Medium |
| Osteitis Fibrosa | High | Normal | Normal to High |
| Adinamic Bone Disease | Low | Normal | Low to Normal |
| Mixed Osteopathy | Normal to High | Abnormal | Low to Normal |
| Osteoporosis | Normal | Normal | Low |
Uremic osteoporosis and bone quality. Advanced stages of CKD are characterized by skeletal fragility, with a risk of fractures four to six-fold higher than age and gender-matched controls without CKD [6]. In these patients impaired bone strength and bone quality assert a certain relation between CKD and osteoporosis.
The pathophysiology is complex, consisting of a mixture of classic osteoporosis, drug-induced bone disorders, and CKD-MBD syndrome [6]. Firstly, primary osteoporosis (age and sex-related or due to other classic factors) could play a more prominent role in CKD bone fragility than previously recognized, impacting early in the course of the disease. Second, a multitude of drugs may cause negative bone effects (e.g., steroids, loop diuretics, heparin, proton pump inhibitors or vitamin K antagonists) but also poor levels of nutrients and vitamins (e.g., vitamin D and vitamin K) are recently recognized to be associated with osteoporosis and fracture risk [7,8]. Third, in CKD patients, bone strength and quality are impaired by the accumulation of uremic toxins, such as indoxyl sulfate, p-cresyl sulfate, and advanced glycation end products (AGE), that create oxidative stress causing impairment, especially in material properties [4,9]. Bone quality is the combination of both structural properties, such as cortical and trabecular microarchitecture, and material properties, such as mineral composition and collagen type I crosslinking, enabling bone to tolerate load and resist fractures. In uremic bone, there is a prevalence of pathological collagen cross-links and immature crystals [9,10]. Moreover, Hsu et al., highlighted how the uremic environment affects bone quality, particularly through reduced expression of the PTH receptor in osteoblasts and association with skeletal resistance to PTH [11].
Thus, a new concept of ‘uremic’ osteoporosis, characterized by low volume but normal turnover and mineralization, is introduced emphasizing the strict relationship between end-stage renal disease (dialysis) and an increase in bone fractures, presuming a potential role into preventing them through the use of dialytic methods with high removal of uremic toxins (e.g., high flux membrane hemodialysis, hemodiafiltration) [10] (Table 1, Figure 2). BB analysis in future studies and clinical practice should investigate this issue with respect of fracture risks.
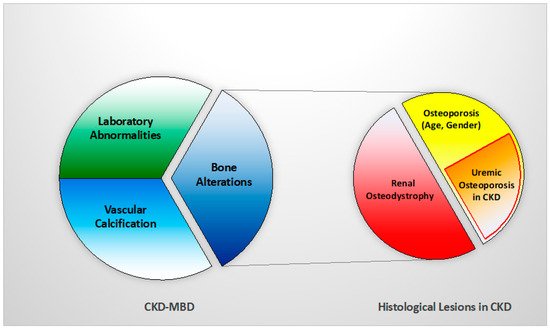
Figure 2. Relationship between CKD-MBD and bone histological patterns in CKD, like renal osteodystrophy and osteoporosis (associated with uremia and/or age-gender, among other factors). This relation impacts bone fragility and fractures susceptibility in CKD patients.
2. ROD Changes over Decades
The epidemiology of ROD has changed for reasons not completely clear, but in part as a consequence of new drugs in the management of MBD and increased survival of patients. High turnover bone disease has long been the predominant type of ROD, while in the last two decades, low turnover disorders have become prominent [12,13]. In particular, Malluche et al., observed this change in 630 bone biopsies of CKD stage 5 patients on maintenance dialysis, performed in Europe or the USA from 2003 to 2008. Distancing from the results of older studies, 58% of patients had a low bone turnover disease. Interestingly, there were racial differences with white patients exhibiting predominantly low turnover (62%), whereas black patients showed mostly normal or high turnover (68%) [12]. Recently, data collected in the Brazilian Registry of Bone Biopsy (REBRABO), which includes BB data of 260 CKD stage 3–5 patients, have not confirmed the trend [14]. Osteitis fibrosa, mixed uremic osteodystrophy, adynamic bone and osteomalacia have been detected in 50%, 25%, 16% and 6% of patients, respectively. In the same cohort, a high prevalence of osteoporosis and aluminium accumulation was also detected.
In the current literature, the actual prevalence of different types of ROD is still debated and this could be related to many factors including epidemiological and clinical differences, such as age and/or ethnicity among CKD patients. Furthermore, differences between health systems could influence the availability of drugs and therapeutic strategies, leading to different types of bone disorders. Histomorphometric data related to CKD patients not on dialysis and to renal transplant recipients are, then, sparse, but some findings show a trend to lower bone turnover for both categories [15,16].
This entry is adapted from the peer-reviewed paper 10.3390/nu14091742
This entry is offline, you can click here to edit this entry!
