Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
The endothelium is considered a real organ, with its own defined structure capable of guaranteeing vascular homeostasis through several functions.
- post-COVID-19
- endothelial function
- nitric oxide
1. Endothelial Cell Homeostasis
The endothelium is considered a real organ, with its own defined structure capable of guaranteeing vascular homeostasis through several functions [1]. Under physiological conditions, ECs are able to respond to a number of hemodynamic and humoral stimuli by producing a wide range of mediators regulating vascular tone, cellular adhesion, coagulation, smooth muscle cell proliferation, and vessel wall inflammation [1]. However, despite all of these being defense mechanisms, these functions might become dysregulated under certain circumstances [2].
To guarantee vascular homeostasis, the endothelium first needs to maintain its intact structure. There are several molecules involved in this process and the main one is vascular endothelial-cadherin (VE-cadherin, also known as CD144), which is a component of endothelial cell-to-cell adherent junctions and a promoter of an optimal organization of ECs cytoskeleton [3]. Moreover, since the endothelium plays a crucial role in controlling immune response, it regulates leucocyte migration into extravascular spaces, defending against infections and promoting tissue repair [4]. ECs show on their surface a number of adhesion molecules (e.g., E-selectin, P-selectin), whose concentration increases in response to proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α). Then, the binding of the leucocytes is reinforced through other adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1, also known as CD54), vascular cell adhesion molecule-1 (VCAM-1, also known as CD106), and integrins [5].
Another key function that the endothelium has is the prevention of thrombosis and the activation of the coagulation cascade, which is a very complex process that involves many factors, among which the most important are platelets and ECs themselves [1]. In fact, several mechanisms can provoke endothelial activation and dysfunction through the loss of ECs structural integrity, leading to the exposure of subendothelial thrombogenic material (e.g., collagen, laminins, nidogens) into the bloodstream, which ultimately activates the coagulation process [6]. To prevent blood clot formation, ECs are able to balance vascular tone by producing several factors that improve dilatation of muscular arteries. Among these, the most important are NO and prostaglandin I2 (PGI2), which combine both antiaggregatory and vasodilator effect [7].
ECs express on their surface a large concentration of molecules involved in the activation of anticoagulant pathways, among which heparan sulphate promotes the anticoagulant effect of antithrombin III (ATIII), while thrombomodulin (TM) stimulates protein C and protein S function [8]. The endothelium can also express plasminogen activators, such as tissue-type plasminogen activator (tPA) and urokinase plasminogen activator (uPA), which enhance the fibrinolytic processes [9][10]. Moreover, ECs can produce adhesion molecules for platelets, such as von Willebrand factor (vWF) and P-selectin, which are exposed on ECs surface upon activation by IL-1β and TNF-α [8]. In turn, platelets produce vascular endothelial growth factor (VEGF), which stimulates the production of tissue factor (TF) from ECs, thus enhancing the activation of coagulation cascade [11].
2. Endothelial Function Assessment
Considering its potential reversibility with targeted strategies, several clinical and laboratory methods have been proposed to evaluate and monitor endothelial function, both in humans and in animal models.
2.1. Clinical Methods
FMD was introduced in clinical research about 20 years ago [12]. In brief, it consists of the measurement of changes in brachial artery diameter as a response to shear stress. In order to evoke this response, a pneumatic cuff placed on the forearm is inflated to a suprasystolic pressure for 5 min. When the cuff is deflated, the increased flow enhances the shear stress on the arterial wall, which stimulates the local production of NO, determining vasodilatation [13]. FMD is a measure of the percentage change of the brachial artery diameter after cuff deflation. Much scientific evidence has demonstrated that FMD represents a reliable method for predicting preclinical CV risk [14][15]. Therefore, recognizing endothelial dysfunction could help physicians in early identification of high-risk patients, giving a more comprehensive assessment of CV risk, which may consequently contribute to better evaluation of personalized CV prevention strategies. The recent identification of age- and sex-specific reference values of FMD in healthy subjects has further confirmed the potential clinical utility of its assessment [16]. On the other hand, despite being a non-invasive and inexpensive method, it has been observed that in the same study population there can be large variations of mean FMD values, depending on some technical variables (e.g., occlusion time, cuff position, patient preparation for examination) and the subsequent operator-dependence [17]. When identifying their reference intervals of FMD, Holder et al. highlighted the need for strict adherence to standardized protocols [16]. However, this may not be sufficient. Thus, the use of dedicated software for real-time edge detection, wall tracking, and shear-rate monitoring has proven to significantly increase reproducibility [18] (Figure 1).
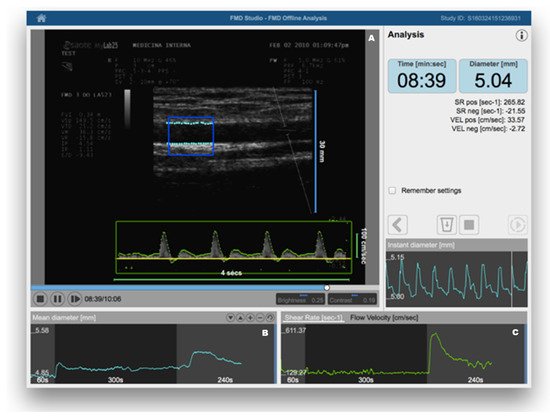
Figure 1. Flow-mediated dilation (FMD) assessment using a Food and Drug Administration (FDA)-cleared software for automatic edge detection (Panel (A)), wall tracking (Panel (B)), and shear-rate monitoring (Panel (C)). Reproduced with permission from Quipu SRL, Pisa, Italy.
Other clinical methods have been proposed for clinical assessment of endothelial function. While venous occlusion plethysmography (VOP) is largely underused because of its invasiveness, laser Doppler flowmetry (LDF) has been used as a non-invasive clinical method for measurement of endothelium-dependent vasodilation in the skin microcirculation [19]. More recently, peripheral artery tonometry (PAT) has become a Food and Drug Administration (FDA)-approved test for an automated assessment of endothelial function [20]. However, although less operator-dependent and highly reproducible, these methods have the disadvantage of being more expensive to use in routine clinical practice and, sometimes, even for research purposes [21].
2.2. Laboratory Methods
Taken together, clinical tests allow measurement of microvascular and macrovascular reactivity, which may fully or partially reflect NO bioavailability. However, a healthy endothelium does not only display a vasodilatory phenotype, depending mainly on NO synthesis [19]. As widely discussed below, under normal circumstances, the endothelium also has an anticoagulant phenotype, which is reflected in the constitutive expression of plasminogen activator inhibitor-1 (PAI-1), vWF, and TF, whose soluble forms can be measured in peripheral blood [22]. The endothelium is also responsible for control of inflammation and oxidative stress, with healthy individuals having low levels of soluble endothelium-derived adhesion molecules or chemokines, including ICAM-1, VCAM-1, E-selectin, P-selectin, VE-cadherin, and monocyte chemotactic protein-1 (MCP-1) [22]. More recently, the levels of various components of the glycocalyx (e.g., syndecan-1, endocan, and heparan sulfate) have been proposed as markers of endothelial injury [23]. Moreover, endothelial progenitor cells (EPCs), reflecting vascular repair capacity, are detected in the blood of healthy individuals, with a progressive reduction with aging and various quantitative and functional alterations in response to acute or chronic pathological stimuli [24]. On the other hand, circulating endothelial cells (CECs) and endothelial microparticles (EMPs) are usually low in healthy individuals, since they reflect the presence of endothelial injury [25]. Overall, a plethora of endothelial biomarkers have been widely used for the identification and characterization of specific endothelial cell types and to test endothelial function both in humans and in animal models.
3. Evidence of Endothelial Dysfunction in COVID-19
From the early stages of the pandemic, it has emerged that endothelial dysfunction could represent the unifying mechanism of COVID-19. Varga et al. were among the first to perform histopathological examinations from autoptic specimens, confirming the presence of endotheliitis in many organs and tissues, with electron microscopy also revealing the presence of SARS-CoV-2 within ECs [26]. The involvement of ECs in the kidneys, lung, heart, skin, and even reproductive system was subsequently highlighted in multiple studies [27][28], suggesting that endothelial damage could represent an important pathogenetic mechanism of respiratory and multiorgan dysfunction [29][30], with a variety of manifestations ranging from CV complications to adverse perinatal outcomes or even erectile dysfunction [31][32].
Using both clinical and laboratory methods for endothelial function assessment, mounting evidence has confirmed the presence of endothelial dysfunction related to SARS-CoV-2 infection. Summarizing the current evidence, a recent meta-analysis showed that several biomarkers of endothelial dysfunction, including vWF, tPA, PAI-1, and soluble thrombomodulin, are significantly associated with increased composite poor outcomes in patients with COVID-19 [33]. Similarly, in addition to these circulatory markers of endothelial function, another meta-analysis showed that high circulating levels of VCAM-1 and E-selectin may be associated with increased COVID-19 severity [34]. Mancuso et al. were among the first to suggest the monitoring of CECs and EPCs as candidate biomarkers of endothelial damage in COVID-19 patients [35]. More recently, increased production of EPCs was also demonstrated during convalescence [36].
Applying the ESC recommendations [37], a number of studies also used clinical methods to test and monitor endothelial function in COVID-19 patients, particularly after the acute phase [38][39][40]. As stated above, most studies employed FMD, given its cost-effectiveness and non-invasiveness, but only a small percentage resorted to dedicated edge-detection software. In the largest study on this topic [38], significantly lower FMD was documented in convalescent COVID-19 patients as compared to controls, confirmed when stratifying the study population according to age and major clinical variables. However, no significant difference was observed between cases and controls in the subgroup analysis on females, in line with the evidence of a disproportionately worse prognosis for male gender [41]. Similar findings were documented among six COVID-19 patients without CV risk when using PAT for endothelial function assessment [42].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10040812
References
- d’Alessandro, E.; Becker, C.; Bergmeier, W.; Bode, C.; Bourne, J.H.; Brown, H.; Buller, H.R.; Ten Cate-Hoek, A.J.; Ten Cate, V.; van Cauteren, Y.J.M.; et al. Thrombo-Inflammation in Cardiovascular Disease: An Expert Consensus Document from the Third Maastricht Consensus Conference on Thrombosis. Thromb. Haemost. 2020, 120, 538–564.
- Libby, P.; Luscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044.
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454.
- Noels, H.; Weber, C.; Koenen, R.R. Chemokines as Therapeutic Targets in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 583–592.
- Mestas, J.; Ley, K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008, 18, 228–232.
- Sturtzel, C. Endothelial Cells. Adv. Exp. Med. Biol. 2017, 1003, 71–91.
- Furchgott, R.F. Endothelium-Derived Relaxing Factor: Discovery, Early Studies, and Identifcation as Nitric Oxide (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1999, 38, 1870–1880.
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815.
- Sawdey, M.S.; Loskutoff, D.J. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J. Clin. Investig. 1991, 88, 1346–1353.
- Levin, E.G.; Loskutoff, D.J. Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J. Cell Biol. 1982, 94, 631–636.
- Nachman, R.L.; Rafii, S. Platelets, petechiae, and preservation of the vascular wall. N. Engl. J. Med. 2008, 359, 1261–1270.
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265.
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12.
- Celermajer, D.S.; Sorensen, K.E.; Bull, C.; Robinson, J.; Deanfield, J.E. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J. Am. Coll. Cardiol. 1994, 24, 1468–1474.
- Anderson, T.J. Prognostic significance of brachial flow-mediated vasodilation. Circulation 2007, 115, 2373–2375.
- Holder, S.M.; Bruno, R.M.; Shkredova, D.A.; Dawson, E.A.; Jones, H.; Hopkins, N.D.; Hopman, M.T.E.; Bailey, T.G.; Coombes, J.S.; Askew, C.D.; et al. Reference Intervals for Brachial Artery Flow-Mediated Dilation and the Relation With Cardiovascular Risk Factors. Hypertension 2021, 77, 1469–1480.
- Bots, M.L.; Westerink, J.; Rabelink, T.J.; de Koning, E.J. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: Effects of technical aspects of the FMD measurement on the FMD response. Eur. Heart J. 2005, 26, 363–368.
- Greyling, A.; van Mil, A.C.; Zock, P.L.; Green, D.J.; Ghiadoni, L.; Thijssen, D.H.; Dilation, T.I.W.G.o.F.M. Adherence to guidelines strongly improves reproducibility of brachial artery flow-mediated dilation. Atherosclerosis 2016, 248, 196–202.
- Klonizakis, M.; Manning, G.; Donnelly, R. Assessment of lower limb microcirculation: Exploring the reproducibility and clinical application of laser Doppler techniques. Skin Pharmacol. Physiol. 2011, 24, 136–143.
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148.
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Luscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767.
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and Biomarkers of Endothelium: When Something Is Rotten in the State. Oxid. Med. Cell. Longev. 2017, 2017, 9759735.
- Celik, T.; Balta, S.; Karaman, M.; Ahmet Ay, S.; Demirkol, S.; Ozturk, C.; Dinc, M.; Unal, H.U.; Yilmaz, M.I.; Kilic, S.; et al. Endocan, a novel marker of endothelial dysfunction in patients with essential hypertension: Comparative effects of amlodipine and valsartan. Blood Press. 2015, 24, 55–60.
- Savoia, C.; Grassi, G. Exercise activity and endothelial function: The uprising role of endothelial progenitor cells in vascular protection. J. Hypertens. 2012, 30, 2083–2084.
- Sabatier, F.; Camoin-Jau, L.; Anfosso, F.; Sampol, J.; Dignat-George, F. Circulating endothelial cells, microparticles and progenitors: Key players towards the definition of vascular competence. J. Cell. Mol. Med. 2009, 13, 454–471.
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418.
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128.
- Kolivras, A.; Dehavay, F.; Delplace, D.; Feoli, F.; Meiers, I.; Milone, L.; Olemans, C.; Sass, U.; Theunis, A.; Thompson, C.T.; et al. Coronavirus (COVID-19) infection-induced chilblains: A case report with histopathologic findings. JAAD Case Rep. 2020, 6, 489–492.
- Dou, Q.; Wei, X.; Zhou, K.; Yang, S.; Jia, P. Cardiovascular Manifestations and Mechanisms in Patients with COVID-19. Trends Endocrinol. Metab. 2020, 31, 893–904.
- Kazemi, S.; Pourgholaminejad, A.; Saberi, A. Stroke Associated with SARS-CoV-2 Infection and its Pathogenesis: A Systematic Review. Basic Clin. Neurosci. 2021, 12, 569–586.
- Pathirathna, M.L.; Samarasekara, B.P.P.; Dasanayake, T.S.; Saravanakumar, P.; Weerasekara, I. Adverse Perinatal Outcomes in COVID-19 Infected Pregnant Women: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 203.
- Delli Muti, N.; Finocchi, F.; Tossetta, G.; Salvio, G.; Cutini, M.; Marzioni, D.; Balercia, G. Could SARS-CoV-2 infection affect male fertility and sexuality? APMIS, 2022; in press.
- Andrianto; Al-Farabi, M.J.; Nugraha, R.A.; Marsudi, B.A.; Azmi, Y. Biomarkers of endothelial dysfunction and outcomes in coronavirus disease 2019 (COVID-19) patients: A systematic review and meta-analysis. Microvasc. Res. 2021, 138, 104224.
- Lampsas, S.; Tsaplaris, P.; Pantelidis, P.; Oikonomou, E.; Marinos, G.; Charalambous, G.; Souvaliotis, N.; Mystakidi, V.C.; Goliopoulou, A.; Katsianos, E.; et al. The Role of Endothelial Related Circulating Biomarkers in COVID-19. A Systematic Review and Meta-analysis. Curr. Med. Chem. 2021.
- Mancuso, P.; Gidaro, A.; Gregato, G.; Raveane, A.; Cremonesi, P.; Quarna, J.; Caccia, S.; Gusso, L.; Rusconi, S.; Giacomelli, A.; et al. Circulating endothelial progenitors are increased in COVID-19 patients and correlate with SARS-CoV-2 RNA in severe cases. J. Thromb. Haemost. 2020, 18, 2744–2750.
- Poyatos, P.; Luque, N.; Eizaguirre, S.; Sabater, G.; Sebastian, L.; Albesa, I.F.; Peracaula, M.; Boixade, M.; Orriols, R.; Tura-Ceide, O. Post-COVID-19 patients show an increased endothelial progenitor cell production. Transl. Res. 2022, 243, 14–20.
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184.
- Ambrosino, P.; Calcaterra, I.; Molino, A.; Moretta, P.; Lupoli, R.; Spedicato, G.A.; Papa, A.; Motta, A.; Maniscalco, M.; Di Minno, M.N.D. Persistent Endothelial Dysfunction in Post-Acute COVID-19 Syndrome: A Case-Control Study. Biomedicines 2021, 9, 957.
- Ergul, E.; Yilmaz, A.S.; Ogutveren, M.M.; Emlek, N.; Kostakoglu, U.; Cetin, M. COVID 19 disease independently predicted endothelial dysfunction measured by flow-mediated dilatation. Int. J. Cardiovasc. Imaging 2022, 38, 25–32.
- Paneroni, M.; Pasini, E.; Vitacca, M.; Scalvini, S.; Comini, L.; Pedrinolla, A.; Venturelli, M. Altered Vascular Endothelium-Dependent Responsiveness in Frail Elderly Patients Recovering from COVID-19 Pneumonia: Preliminary Evidence. J. Clin. Med. 2021, 10, 2558.
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581.
- Cimino, G.; Vizzardi, E.; Calvi, E.; Pancaldi, E.; Pascariello, G.; Bernardi, N.; Cersosimo, A.; Amore, L.; Inciardi, R.M.; Raddino, R.; et al. Endothelial dysfunction in COVID-19 patients assessed with Endo-PAT2000. Monaldi Arch. Chest Dis. 2022.
This entry is offline, you can click here to edit this entry!
