Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Autism spectrum disorders (ASD) are pervasive neurodevelopmental disorders that include a variety of forms and clinical phenotypes. This heterogeneity complicates the clinical and experimental approaches to ASD etiology and pathophysiology. To date, a unifying theory of these diseases is still missing. With the recent acknowledgement of the cerebellar contribution to cognitive functions and the social brain, its involvement in ASD has become unmistakable, though its extent is still to be elucidated.
- cerebellum
- autism spectrum disorders
- social brain
- excitatory/inhibitory balance
1. Neural Bases for Impaired Social Cognition in Autism Spectrum Disorders (ASD)
In humans, focal brain lesions and social task-based fMRI have largely contributed to identifying a network of brain regions (called “the social brain”) implicated in social cognition [1][2][3][4][5]. The primary regions of the social brain include the medial prefrontal cortex (mPFC) [6][7], the temporoparietal junction (TPJ), the posterior superior temporal sulcus (pSTS), the inferior frontal gyrus, the anterior cingulate cortex (ACC) [8], and the anterior insula (AI) [9] (Figure 1). Moreover, the hippocampal formation, the ventral tegmental area (VTA), the nucleus accumbens (NAcc), the amygdala, and the cerebellum are highly connected with the social network structure, acting as important functional hubs [10][11][12][13][14][15][16]. In ASD subjects, several identified a combination of atypical structural and functional features in these areas. Structurally, cortical and subcortical measurements in ASD postmortem brain tissue, primarily in frontal and temporal cortices and the amygdala, described an aberrant organization, such as small cell size and increased packing density [17][18], white matter volume increase [19], decreased cortical thickness [20], and more numerous and narrower minicolumns [21]. Functionally, a growing number used fMRI to examine changes in intrinsic functional connectivity (FC) of specific brain regions and circuits [22][23] between individuals with ASD and normally developing controls. In most cases, FC analysis indicated that ASD subjects exhibit long-range under-connectivity and local over-connectivity [24][25][26][27][28][29][30][31]. Long-range under-connectivity between prefrontal cortex (PFC) and posterior brain regions were most often reported [24][25][26][32][33], but it was also described in other regions, as between the amygdala and temporal cortex [34], the supplementary motor areas and the thalamus [35], the PFC and premotor and somatosensory cortices [36], and among the PFC, amygdala, and hippocampus [37]. However, some reported increased FC among individuals with ASD [37]. Specifically, long-range over-connectivity was discovered within thalamocortical [38], striatocortical [39], and corticocortical circuits [40]. In contrast, local over-connectivity in ASD is less solidly determined. For instance, local over-connectivity was found in ASD in the extrastriate cortex, frontal and temporal regions, amygdala, and parahippocampal gyri [41][42][43][44][45]. Other however, reported a reduction of local connectivity, principally in the fusiform face area and in the somatosensory cortex [46][47], or a combination of both patterns [48]. Several experimental evidence suggested a higher excitatory/inhibitory (E/I) ratio as a possible correlate for local over-connectivity [49][50][51], for example, through an increased glutamatergic or reduced GABAergic signaling [52][53]. Cortical GABAergic neurons are thought to control the functional integrity and segregation of minicolumns via lateral inhibition [54]. Casanova and colleagues [21] found significant differences between frontal and temporal cortices of ASD and typically developing individuals in the number of minicolumns, in the horizontal space between minicolumns, and their internal structure. Ultimately, minicolumns were more numerous, smaller, and less compact in their cellular configuration. Mechanisms underlying this deficit are still unknown. Moreover, GABAergic neurotransmission is involved in generating gamma-band oscillatory activity [55]. Gamma-band oscillations are involved in a wide range of cognitive processes from the perception of gestalt [56] to selective attention [57][58][59] and working memory [60][61]. Magnetoencephalography (MEG) and electroencephalography (EEG) have reported correlations between gamma-band oscillatory activity and ASD severity as measured by the Social Responsiveness Scale [62][63][64]. It should also be considered that an unbalanced E/I ratio might be amplified by delayed brain development, resulting in retardation of synaptogenesis, pruning, and myelination [65][66][67]. Lastly, strong evidence is also reported for alterations in glutamatergic signaling pathways in ASD, involving metabotropic glutamate receptor 5 (mGluR5) upregulation and genetic aberrations associated with NMDA receptors [68][69]. However, the scenario is much more complicated, with both increases and decreases in glutamate-mediated signaling reported in association with the ASD phenotype [70]. Overall, the above-presented data lend support to models hypothesizing well-defined neural substrates of social cognition and propose specific neural bases that may govern social cognitive impairments in ASD. By contrast, further investigations are needed to better understand the complex interactions between social brain areas, connectivity, frequency bands, and physiological aspects (for example, roles of specific cell types, maturational processes, receptors) and how they relate to different cognitive processes.

Figure 1. Anatomy of the social brain. The main brain areas involved in the “social brain” are reported in the medial (left) and lateral (right) schematic view of the human brain: medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), temporo-parietal junction (TPJ), posterior superior temporal sulcus (pSTS), inferior frontal gyrus (IFG), and anterior insula (AI). The main regions connected to the “social brain” are reported in grey: hippocampus (hip), amygdala (am), ventral tegmental area (VTA), nucleus accumbens (NAcc), and cerebellum.
2. Cerebellar Involvement in ASD
2.1. Cerebellar Circuit Microanatomy
The cerebellum, meaning “little brain” in Latin, has been historically considered a subcortical motor structure that controls the coordination of voluntary movements, balance, posture, and muscle tone. Furthermore, it contributes to different forms of motor learning. There is now robust evidence that the cerebellum may be related to a variety of cognitive and emotional functions such as language, attention, fear, and pleasure responses [71][72][73][74]. The cerebellum is composed of tightly folded layers of grey matter forming the cerebellar cortex, with the white matter underneath surrounding four deep cerebellar nuclei (DCN) [75]. The cerebellar cortex is organized into three layers. The outer molecular layer (ML) is composed of two types of inhibitory neurons: stellate (SCs) and basket cells (BCs). The Purkinje cell layer consists of a large pear-shaped Purkinje cell (PC) soma monolayer. The inner granular layer is composed of excitatory granule cells (GrCs) and inhibitory Golgi cells (GoCs). The primary input pathways entering the cerebellum are the mossy fibers (MFs) and climbing fibers (CFs). In the granular layer, MFs directly synapse on the dendrites of GrCs, whose axons ascend toward the ML, where they bifurcate to form T-shaped branches named parallel fibers (PFs) [76][77][78]. PCs receive excitatory input from PFs and CFs, which originate in the inferior olive (IO) [79] and project their axons to DCN neurons. DCN neurons provide the final output of the cerebellum by integrating inhibitory and excitatory inputs from PC axons, MF, and CF collaterals, respectively [80][81]. The activity of PCs is modulated by three types of inhibitory interneurons that are activated by PFs and classified into two main types: BCs and SCs, which are found in the ML, and GoCs, located in the granular layer. Specifically, BCs are found in the deep ML and their axons form pericellular nests in close proximity to PC soma as well as specialized terminals known as pinceaux surrounding the initial segment of PC axons. SCs are located in the upper ML and their axons terminate on the shafts of PC dendrites [82][83]. GoCs receive excitatory synaptic input from MFs on the basal dendrites and PFs on the apical dendrites [84][85], and their axons make inhibitory synapses with GrCs [86][87]. Thus, GoC activity indirectly affects PC output by modulating GrC discharge [82][88] (Figure 2).
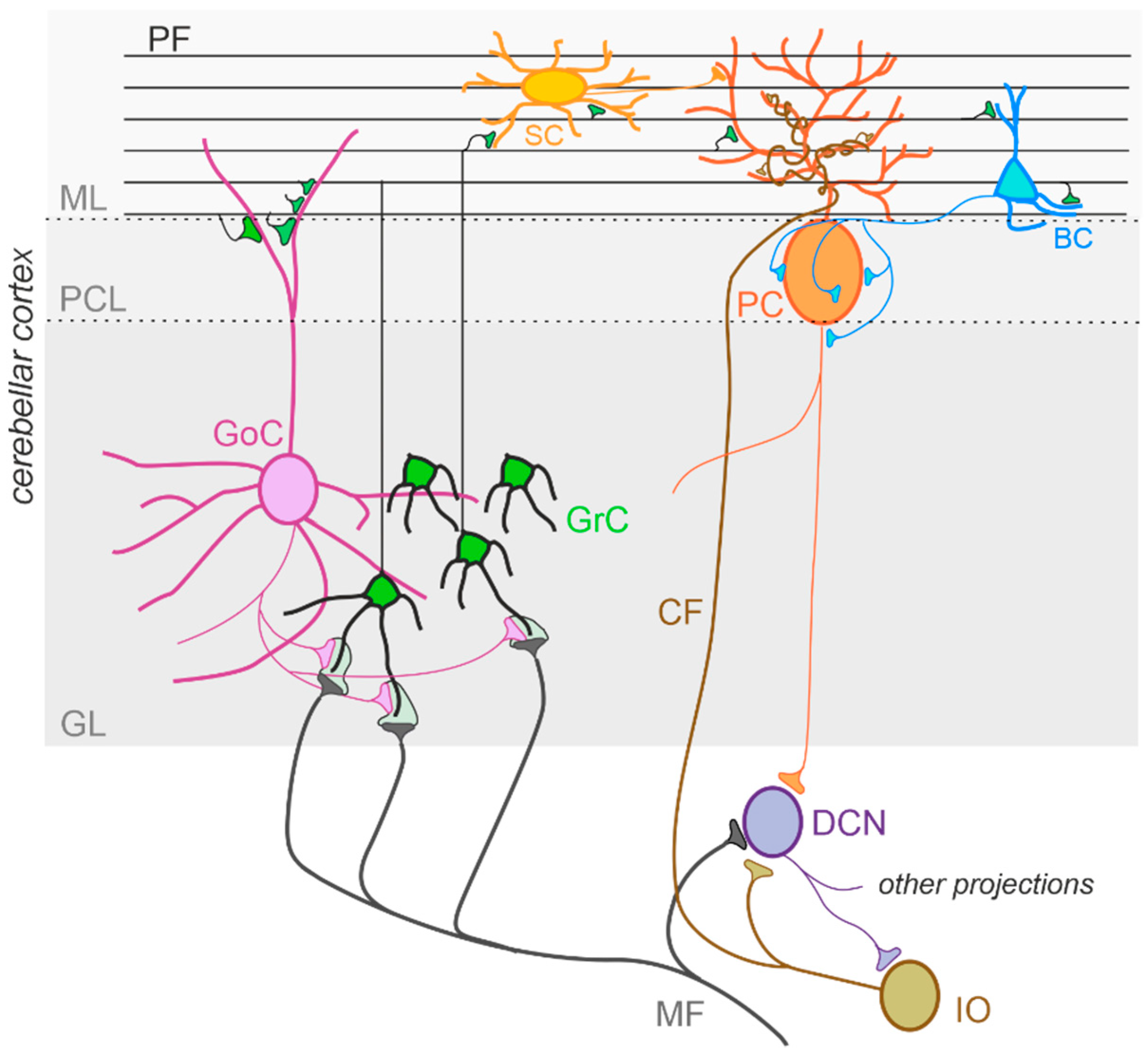
Figure 2. The cerebellar circuit. Schematic representation of the main components of the cerebellar circuit. The inputs are provided by mossy fibers (MF) and climbing fibers (CF), these latter originating in the inferior olive (IO). Both inputs send collaterals to the deep cerebellar nuclei (DCN) before entering the cerebellar cortex. Granule cells (GrC) and Golgi cells (GoC) are the main neuronal types present in the granular layer (GL) of the cerebellar cortex. GrC axons reach the molecular layer (ML) where they bifurcate originating the parallel fibers (PF). The inhibitory interneurons in the ML are stellate cells (SC) and basket cells (BC), which inhibit Purkinje cells (PC) in the Purkinje cell layer (PCL). The PC provides the output of the cerebellar cortex, inhibiting DCN neurons, which in turn provide the main output of the cerebellar circuit. Notice that DCN project to the IO, generating a loop mediated by the CF.
2.2. Cerebellar Connectivity to Social Brain Areas
Experiments using task-based fMRI and positron emission tomography (PET) revealed that separate regions of the cerebellum are associated with distinct cerebral areas through polysynaptic circuits, forming a functional topography [89][90][91][92]. The sensorimotor cerebellum is represented in the anterior lobe (lobules I-V) and lobule VIII, while the cognitive cerebellum comprises the posterior lobe (lobules VI and VII), including hemispheric extensions (CrusI/CrusII) [92]. Finally, the posterior vermis and hemispheres represent the limbic cerebellum [92][93][94]. The DCN send direct projections to the ventrolateral (VL) and the intralaminar thalamic nuclei, particularly the dorsomedial (MD) nucleus [95]. The VL nucleus, classically known as an integrative center for sensorimotor transformations, targets the primary motor cortex (M1) [10][96][97], whereas the MD nucleus, like other intralaminar nuclei, has widespread cortical projections including the medial prefrontal cortex (mPFC) (Figure 3A) and the superior temporal sulcus [98][99][100][101][102]. Past anatomical demonstrated that the cerebellum is interconnected with parts of the limbic system, including the hippocampus, amygdala, and cingulate cortex (Figure 3B) [103][104]. Recently, Bohne and colleagues [105] reported an elegant tracing one identifying a new cerebellar-hippocampal connection via the VL thalamic nucleus in mice. In support of this finding, unilateral removal of the cerebellar hemispheres [106] or PC signaling deficits [107] determined an impairment in hippocampal-based behavioral tasks as goal-directed navigation tests. Furthermore, monosynaptic projections originate in the hippocampus to primarily target the PFC in rodents and primates [108][109]. Several shreds of evidence obtained using different methodologies show that the cerebellum and amygdala are connected [110]. For example, Sang and colleagues found functional connectivity between cerebellar lobules I-V and the amygdala, analyzing resting-state fMRI in healthy young adults [111]. Heath and Harper, recording evoked potentials or using histological tract-tracing, showed connections between DCN and amygdala in cats and monkeys [112]. Finally, Morris and colleagues found amygdala and cerebellum coactivation during the presentation of facial expressions in human subjects [113]. The cerebellum is also connected with the cingulate cortex indicating its involvement in motivational and emotional processing [114]. Early animal ones showed electrophysiological responses in the ACC following electrical stimulation of the vermis area [112][115]. These results were confirmed almost forty years later by Krienen and Buckner using resting-state fMRI in young adults, showing that CrusI and anterior cingulate cortex were functionally connected [116]. Lastly, electrical stimulation of DCN was reported to evoke dopamine release in the mPFC in rodents [117][118][119]. Cerebellar modulation of dopamine release onto the mPFC could be mediated by two separate neuronal pathways originating from the DCN. The first one activates the mesocortical dopaminergic pathway via reticulo-tegmental nuclei (RTN), which, in turn, project to pedunculopontine nuclei (PPT) and then directly stimulate VTA dopaminergic cells that send their axons to the mPFC [103][120][121][122][123]. The second is by modulation of mesocortical dopaminergic release via glutamatergic afferents originating in the thalamic nuclei (VL and MD) [11][124][125] (Figure 3B). More intriguingly, Recently has shown, using optogenetic manipulation, the existence of a direct cerebellum-VTA pathway suggesting a prominent role of the cerebellum in modulating social behavior [126]. The primary cerebellar connections reported above are summarized in Figure 3. Altogether, these findings propose that dysfunctions described within the cerebral cortical network, usually associated with social features of ASD, could be at least partly related to an impaired connectivity between the cerebellum and key social brain areas.

Figure 3. Cerebellar connectivity to other brain areas. The cerebellum is one of the most interconnected structures in the brain. (A) Schematic representation of the mouse brain and the main cerebellar connections thought to be relevant for its role in ASD. DCN, deep cerebellar nuclei; PN, pontine nuclei (including reticulo-tegmental nuclei and pedunculopontine nuclei); VTA, ventral tegmental area; am, amygdala; hip, hippocampus; VL, ventrolateral thalamic nucleus; MD, mediodorsal thalamic nucleus; M1, primary motor cortex; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex. (B) Same representation as in (A), showing the pathways involving the limbic system. (C) Same representation as in (A), showing the connections involved in the mesocortical dopaminergic pathways, regulating mPFC activity modulation.
2.3. Cerebellar Structural Abnormalities in ASD
The cerebellum is the brain structure most constantly found abnormal in ASD, and an increased risk for ASD is dependently associated with cerebellar damage [127][128][129][130][131]. Early anatomical ones examining postmortem ASD brain tissue reported a significant reduction in the number of PCs in the lateral hemisphere compared with the medial vermis [132][133]. In subsequent years, the reduction in PC density has been widely documented (about 75% of ASD were reported [134][135][136][137]). Fatemi and colleagues [138] found a reduction in PC size in about 25% of ASD. Additionally, a variable decrease in GrCs numerosity was reported [139], while the molecular interneurons were preserved [140]. Animal models of spontaneous cerebellar mutations are frequently characterized by PC loss, often showing a failure in the regression of multiple innervations of PCs by CFs, with each PC receiving up to four CF inputs [141][142][143] instead of a single one [144]. A similar PC hyper-innervation due to decreased PC number has not yet been described in human ASD brains, but it could provide support to IO neurons, which are unaffected in number [145][146][147]. Concerning the DCN, the neurons presented an enlarged size at a younger age, while older ASD cerebella showed abnormally smaller neurons, also reduced in number in fastigial and interposed nuclei [139][146]. MRI has emerged as a powerful tool for visualization and diagnostics of neuroanatomical abnormalities in ASD since its inception in the 1980s. Nevertheless, many results are contradictory due to the heterogeneity of underlying causes and the complexity of this disorder. Structural MRI in ASD patients described a reduction in the size of several regions of the cerebellum including the vermis, particularly the lobules VI and VII [146][148][149][150][151][152]. In contrast, Piven and colleagues [153] did not confirm these findings but revealed an enlarged cerebellar volume in ASD patients. Interestingly, the cerebellar volume was proportionally scaled to the total brain volume [154]. Further findings included an enlargement in cerebellar white matter volume and a reduction in the grey/white matter ratio [155][156]. In conclusion, the abnormalities of cerebellar structural integrity may be considered as significant predictive factors of ASD and cause differences in functional connectivity reported in ASD young adults (see paragraph below).
2.4. Cerebellar Functional Abnormalities in ASD
Children and young adults with ASD, using resting-state FC (rsFC) techniques, documented a general cerebro-cerebellar over-connectivity [157]. However, both under- and over- connectivity have been observed depending on the brain regions investigated. For example, rsFC were increased between non-motor areas of the cerebellum (lobules VI and CrusI) and sensorimotor cerebral cortical regions, such as premotor and primary motor cortices, somatosensory temporal cortex, and occipital lobe; and decreased in cerebro-cerebellar circuits involving language and social interaction, particularly between CrusI/II and PFC, posterior parietal cortex, and the inferior/middle temporal gyrus [127][157][158]. It should be noted that no specific correlation between FC and behavioral profiles in individuals with ASD has been established [42][159][160][161], although novel findings reported abnormalities in FC related to ASD symptom severity [162][163]. To date, very few addressed the FC between the cerebellum and cortical regions, focusing on motor task performance in ASD. For example, during self-paced sequential finger tapping, fMRI in children with ASD did not display the activation in the lobules IV/V and in the anterior cerebellum present in typically developing groups [35]. Furthermore, Jack and Morris [164] investigated coordinated activity between the neocerebellum (particularly CrusI) and pSTS during a task that requires perception and use of information about others’, and remarkably found stronger CrusI–pSTS connectivity positively associated with mentalizing ability, in young adults with ASD. Therefore, together with the structural data described above, these findings are consistent with the idea that ASD is a disorder characterized by abnormalities in cerebellum-cerebral functional connectivity, which could be related to symptom severity.
2.5. Cerebellar Neurochemical Abnormalities in ASD
Neurochemical research has progressed in the last 20 years and has produced promising results. For example, reelin expression was reduced in the cerebellum of ASD individuals [165]. This glycoprotein regulates proper cortex lamination and neuronal migration during development and adult life, sustaining cell signaling and synaptic function. Furthermore, serotonin concentration is also altered in the ASD cerebellum. Specifically, Chugani and colleagues [166][167], using PET scanning with a tracer for serotonin synthesis in ASD young adults, reported reduced serotonin levels in the thalamus and the frontal cortex associated with increased serotonin concentration in DCN. Serotonin is well known for its role in neurodevelopment, regulating cell migration and proliferation [168], neurite outgrowth, and neuronal survival [169] as well as synaptogenesis [170]. Therefore, aberrant serotoninergic neuromodulation of dentatothalamocortical pathway connecting the cerebellum with social structures could compromise cognitive and behavioral maturation in ASD. Additionally, a reduced expression in PCs of glutamic acid decarboxylase 67 (GAD67) mRNA, an essential enzyme for converting glutamate to GABA, is a consistent finding in the postmortem cerebellum of ASD patients [171][172]. Conversely, a higher expression of GAD67 mRNA in cerebellar molecular layer interneurons was observed [173], suggesting the existence of an upregulation mechanism to counterbalance the altered inhibition of DCN by PCs. Interestingly, the larger-sized subpopulation of GABAergic neurons in the DCN, which project specifically to the IO [96][174], were reported to exhibit a reduction in GAD65 mRNA expression [175]. Thus, GABAergic neurotransmission alteration in DCN could profoundly affect olivary oscillations and subsequently affect the timing of PC activity (Figure 4). To date, accumulating evidence hints for the hypothesis that core features of ASD emerge from disturbances in the E/I balance within neural circuits [49][53][145][176]. In conclusion, the above findings highlight the role of reelin, serotonin concentration, GABAergic neurotransmission and GAD enzymes in ASD. However, more investigations are needed to better evaluate the mechanisms underlying E/I balance.
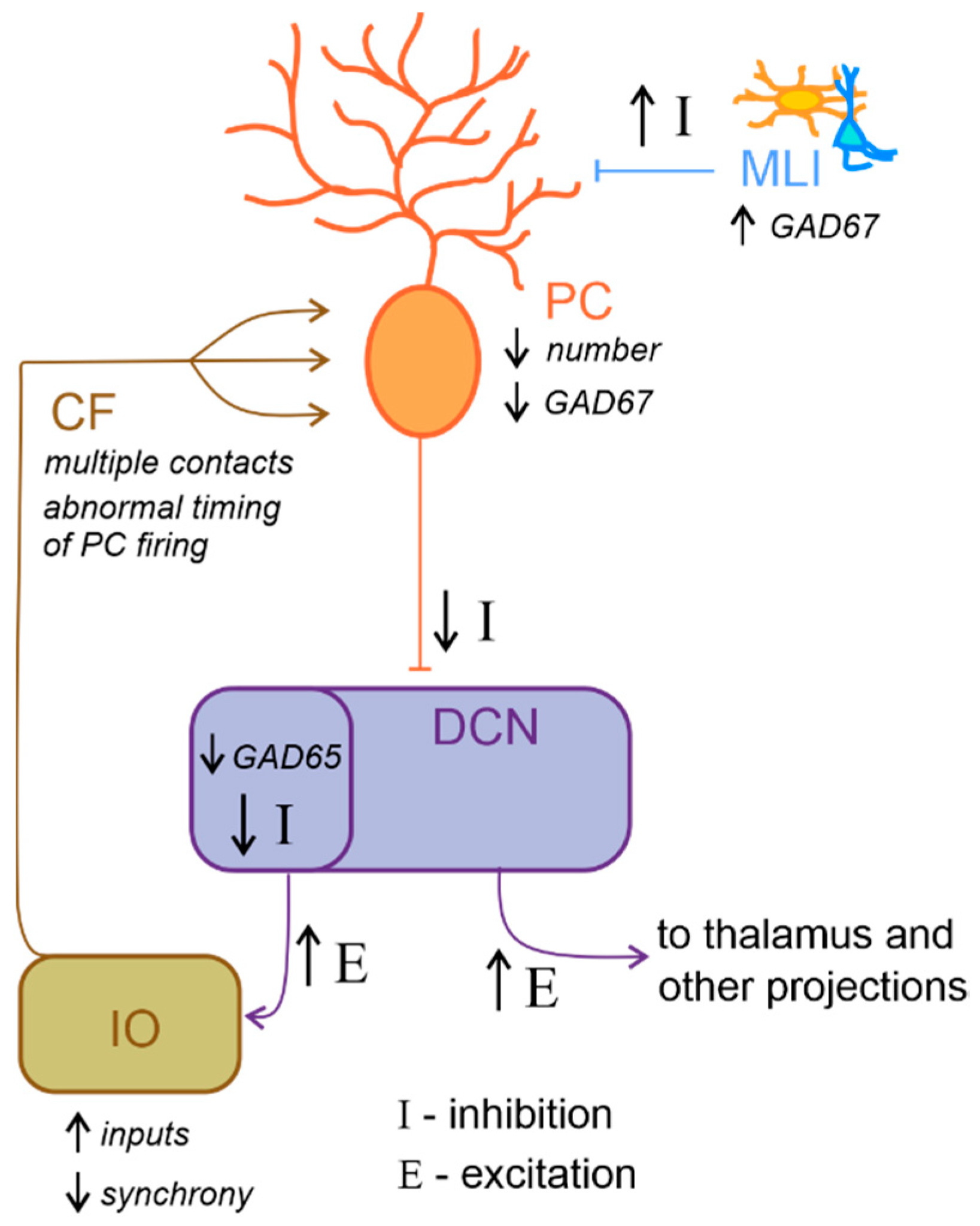
Figure 4. Altered excitatory/inhibitory balance in the cerebellum-inferior olive loop in ASD. Schematic representation of the main alterations described in the cerebellum-inferior olive circuit in ASD, as described in the main text. Briefly, Purkinje cells (PC) are reduced in number and show a decreased level of GAD67 mRNA expression, while molecular layer interneurons (MLI) show an increased inhibition over PC. These would likely determine a decrease in the inhibition (I) over deep cerebellar nuclei (DCN) neurons. DCN disinhibition would increase the excitatory (E) level increasing the output towards the thalamus and other brain regions. Concerning the loop with the inferior olive (IO), DCN neurons which project to this area show decreased GAD65 mRNA expression levels, thus resulting in a decreased inhibition over IO neurons, likely increasing the excitatory inputs and decreasing synchronicity. In ASD, multiple climbing fibers (CF) impinging on the same PC were described. Together with the alterations in IO activity, this anatomical abnormality likely contributes to impair the timing of PC spiking activity.
2.6. Cerebellar Inflammation in ASD
Cells of the immune system and their products are able to directly regulate neuronal function, cell migration, proliferation, adhesion, differentiation [177], and synapse formation and plasticity [178]. Thus, they play crucial roles in modulating neuronal circuits that constitute the basis for human social cognition and behavior [130]. Immune profile alterations have been described in ASD patients [179][180][181][182][183] and may contribute to the disorders. Postmortem brain tissue from ASD individuals often shows persistent neuroinflammation [184]. Specifically, in cerebellar tissue samples, aberrant microglia and astrocyte activation was detected in combination with a massive monocyte and macrophage accumulation, particularly in the granular layer and white matter [184]. These findings correlated with marked histological abnormalities including decreased numbers of PCs and GrCs together with reduced numbers of axons within the PC layer [180][184]. Moreover, in ASD patients, increased levels of many cytokines and chemokines were described in the brain and cerebrospinal fluid, precisely, interleukin (IL)-6, tumor necrosis factor alpha (TNF-α), transforming growth factor beta 1 (TGFβ1), and C-C motif ligand 2 and 17 (CCL2 and CCL17) in the cerebellum [184][185][186][187]. Furthermore, antibodies against cerebellar proteins have also been characterized in ASD individuals and are strongly associated with impairments in behaviors, in particular deficits in social interactions and communication [188][189][190][191]. The antigenic target of these antibodies has not yet been precisely identified but robust specific reactivity was shown against cerebellar GABAergic interneurons, including Golgi cells [189][192]. Whether these antibodies alter activity of its target neurons or mark them for destruction by phagocytes requires further investigation. Remarkably, Black and Tan BRachyury (BTBR) inbred mice were identified only fifteen years ago as showing strong and consistent autism-relevant behaviors, including reduced social interactions, impaired play, low exploratory activity, unusual vocalizations and high anxiety [193][194]. These mice show a number of immunological abnormalities, many of which were described in postmortem brains of ASD subjects [195][196][197][198]. They are characterized by elevated cytokine levels in the brain, and an increased proportion of microglial cells. In particular, among the brain regions that Heo and colleagues [195] examined, the cerebellum exhibited significantly higher expression of IL-33, IL-18 and IL-6 in BTBR mice than in control, suggesting that it could be a crucial area for neuroinflammation in humans with ASD. Finally, a recent one revealed an abnormal cerebellar development (enhanced foliation and PC hypotrophy with altered dendritic spine formation) concomitant with the progression of motor impairments in BTBR mice [199]. In summary, although there is a growing body of evidence supporting the relationship between cytokine alterations and ASD, systematic and large scale investigations are needed to better clarify the role of cerebellar inflammation on the emergence of ASD and the contribution to its etiological heterogeneity.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23073894
References
- Redcay, E.; Moraczewski, D. Social cognition in context: A naturalistic imaging approach. NeuroImage 2020, 216, 116392.
- Doruyter, A.; Groenewold, N.A.; Dupont, P.; Stein, D.J.; Warwick, J. Resting-state fMRI and social cognition: An opportunity to connect. Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2627.
- Lee, K.-H.; Brown, W.H.; Egleston, P.N.; Green, R.D.; Farrow, T.F.; Hunter, M.D.; Parks, R.W.; Wilkinson, I.D.; Spence, S.A.; Woodruff, P.W. A Functional Magnetic Resonance Imaging Study of Social Cognition in Schizophrenia During an Acute Episode and After Recovery. Am. J. Psychiatry 2006, 163, 1926–1933.
- Maggio, M.G.; Maresca, G.; Stagnitti, M.C.; Anchesi, S.; Casella, C.; Pajno, V.; De Luca, R.; Manuli, A.; Calabrò, R.S. Social cognition in patients with acquired brain lesions: An overview on an under-reported problem. Appl. Neuropsychol. Adult 2020. ahead of print.
- Sokolov, A.A. The Cerebellum in Social Cognition. Front. Cell. Neurosci. 2018, 12, 145.
- Amodio, D.M.; Frith, C.D. Meeting of minds: The medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006, 7, 268–277.
- Beer, J.S.; Ochsner, K.N. Social cognition: A multi level analysis. Brain Res. 2006, 1079, 98–105.
- Mar, R.A. The Neural Bases of Social Cognition and Story Comprehension. Annu. Rev. Psychol. 2011, 62, 103–134.
- Kipps, C.M.; Duggins, A.J.; McCusker, E.A.; Calder, A.J. Disgust and Happiness Recognition Correlate with Anteroventral Insula and Amygdala Volume Respectively in Preclinical Huntington’s Disease. J. Cogn. Neurosci. 2007, 19, 1206–1217.
- Kelly, R.M.; Strick, P.L. Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J. Neurosci. 2003, 23, 8432–8444.
- Middleton, F.; Strick, P.L. Cerebellar Projections to the Prefrontal Cortex of the Primate. J. Neurosci. 2001, 21, 700–712.
- Passingham, R.E.; Stephan, K.E.; Kötter, R. The anatomical basis of functional localization in the cortex. Nat. Rev. Neurosci. 2002, 3, 606–616.
- Schmahmann, J.D. An Emerging Concept. The cerebellar contribution to higher function. Arch. Neurol. 1991, 48, 1178–1187.
- Laurita, A.; Spreng, N. The Hippocampus and Social Cognition. In The Hippocampus from Cells to Systems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 537–558.
- Settell, M.; Testini, P.; Cho, S.; Lee, J.H.; Blaha, C.D.; Jo, H.J.; Lee, K.H.; Min, H.-K. Functional Circuitry Effect of Ventral Tegmental Area Deep Brain Stimulation: Imaging and Neurochemical Evidence of Mesocortical and Mesolimbic Pathway Modulation. Front. Neurosci. 2017, 11, 104.
- Gunaydin, L.A.; Grosenick, L.; Finkelstein, J.C.; Kauvar, I.V.; Fenno, L.E.; Adhikari, A.; Lammel, S.; Mirzabekov, J.J.; Airan, R.D.; Zalocusky, K.A.; et al. Natural Neural Projection Dynamics Underlying Social Behavior. Cell 2014, 157, 1535–1551.
- Bauman, M.L.; Kemper, T.L. Neuroanatomic observations of the brain in autism: A review and future directions. Int. J. Dev. Neurosci. 2005, 23, 183–187.
- Courchesne, E.; Mouton, P.R.; Calhoun, M.E.; Semendeferi, K.; Ahrens-Barbeau, C.; Hallet, M.J.; Barnes, C.C.; Pierce, K. Neuron Number and Size in Prefrontal Cortex of Children with Autism. JAMA 2011, 306, 2001–2010.
- Casanova, M.F. White matter volume increase and minicolumns in autism. Ann. Neurol. 2004, 56, 453.
- Richter, J.; Henze, R.; Vomstein, K.; Stieltjes, B.; Parzer, P.; Haffner, J.; Brandeis, D.; Poustka, L. Reduced cortical thickness and its association with social reactivity in children with autism spectrum disorder. Psychiatry Res. Neuroimaging 2015, 234, 15–24.
- Casanova, M.F.; Buxhoeveden, D.P.; Switala, A.E.; Roy, E. Minicolumnar pathology in autism. Neurology 2002, 58, 428–432.
- Biswal, B.B. Resting state fMRI: A personal history. NeuroImage 2012, 62, 938–944.
- Buckner, R.L.; Krienen, F.M.; Yeo, B.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013, 16, 832–837.
- Just, M.A.; Cherkassky, V.L.; Keller, T.A.; Minshew, N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain 2004, 127, 1811–1821.
- Just, M.A.; Keller, T.A.; Malave, V.L.; Kana, R.K.; Varma, S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neurosci. Biobehav. Rev. 2012, 36, 1292–1313.
- Courchesne, E.; Pierce, K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005, 15, 225–230.
- Kana, R.K.; Keller, T.A.; Minshew, N.J.; Just, M.A. Inhibitory Control in High-Functioning Autism: Decreased Activation and Underconnectivity in Inhibition Networks. Biol. Psychiatry 2007, 62, 198–206.
- Just, M.A.; Cherkassky, V.L.; Keller, T.A.; Kana, R.K.; Minshew, N.J. Functional and Anatomical Cortical Underconnectivity in Autism: Evidence from an fMRI Study of an Executive Function Task and Corpus Callosum Morphometry. Cereb. Cortex 2007, 17, 951–961.
- Assaf, M.; Jagannathan, K.; Calhoun, V.D.; Miller, L.; Stevens, M.; Sahl, R.; O’Boyle, J.G.; Schultz, R.T.; Pearlson, G.D. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage 2010, 53, 247–256.
- Wass, S. Distortions and disconnections: Disrupted brain connectivity in autism. Brain Cogn. 2011, 75, 18–28.
- Rane, P.; Cochran, D.; Hodge, S.M.; Haselgrove, C.; Kennedy, D.N.; Frazier, J.A. Connectivity in Autism: A Review of MRI Con-nectivity Studies. Harv. Rev. Psychiatry 2015, 23, 223–244.
- Zikopoulos, B.; Barbas, H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front. Hum. Neurosci. 2013, 7, 609.
- Long, Z.; Duan, X.; Mantini, D.; Chen, H. Alteration of functional connectivity in autism spectrum disorder: Effect of age and anatomical distance. Sci. Rep. 2016, 6, 26527.
- Monk, C.S.; Weng, S.-J.; Wiggins, J.L.; Kurapati, N.; Louro, H.M.; Carrasco, M.; Maslowsky, J.; Risi, S.; Lord, C. Neural circuitry of emotional face processing in autism spectrum disorders. J. Psychiatry Neurosci. 2010, 35, 105–114.
- Mostofsky, S.H.; Powell, S.K.; Simmonds, D.J.; Goldberg, M.C.; Caffo, B.; Pekar, J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain 2009, 132, 2413–2425.
- Lombardo, M.V.; Eyler, L.; Moore, A.; Datko, M.; Barnes, C.C.; Cha, D.; Courchesne, E.; Pierce, K. Default mode-visual network hypoconnectivity in an autism subtype with pronounced social visual engagement difficulties. eLife 2019, 8, e47427.
- Maximo, J.; Cadena, E.J.; Kana, R.K. The Implications of Brain Connectivity in the Neuropsychology of Autism. Neuropsychol. Rev. 2014, 24, 16–31.
- Mizuno, A.; Villalobos, M.E.; Davies, M.M.; Dahl, B.C.; Müller, R.-A. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006, 1104, 160–174.
- Di Martino, A.; Kelly, C.; Grzadzinski, R.; Zuo, X.-N.; Mennes, M.; Mairena, M.A.; Lord, C.; Castellanos, F.; Milham, M.P. Aberrant Striatal Functional Connectivity in Children with Autism. Biol. Psychiatry 2011, 69, 847–856.
- Shih, P.; Shen, M.D.; Öttl, B.; Keehn, B.; Gaffrey, M.S.; Müller, R.-A. Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia 2010, 48, 2931–2939.
- Maximo, J.O.; Keown, C.L.; Nair, A.; Müller, R.-A. Approaches to local connectivity in autism using resting state functional connectivity MRI. Front. Hum. Neurosci. 2013, 7, 605.
- Keown, C.L.; Shih, P.; Nair, A.; Peterson, N.; Mulvey, M.E.; Müller, R.-A. Local Functional Overconnectivity in Posterior Brain Regions Is Associated with Symptom Severity in Autism Spectrum Disorders. Cell Rep. 2013, 5, 567–572.
- Barttfeld, P.; Wicker, B.; Cukier, S.; Navarta, S.; Lew, S.; Sigman, M. A big-world network in ASD: Dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia 2011, 49, 254–263.
- Murphy, E.R.; Foss-Feig, J.; Kenworthy, L.; Gaillard, W.D.; Vaidya, C.J. Atypical Functional Connectivity of the Amygdala in Childhood Autism Spectrum Disorders during Spontaneous Attention to Eye-Gaze. Autism Res. Treat. 2012, 2012, 652408.
- Shih, P.; Keehn, B.; Oram, J.K.; Leyden, K.M.; Keown, C.L.; Müller, R.-A. Functional Differentiation of Posterior Superior Temporal Sulcus in Autism: A Functional Connectivity Magnetic Resonance Imaging Study. Biol. Psychiatry 2011, 70, 270–277.
- Khan, S.; Gramfort, A.; Shetty, N.R.; Kitzbichler, M.G.; Ganesan, S.; Moran, J.M.; Lee, S.M.; Gabrieli, J.D.E.; Tager-Flusberg, H.B.; Joseph, R.M.; et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2013, 110, 3107–3112.
- Coskun, M.A.; Loveland, K.A.; Pearson, D.A.; Papanicolaou, A.C.; Sheth, B.R. Functional Assays of Local Connectivity in the Somatosensory Cortex of Individuals with Autism. Autism Res. 2013, 6, 190–200.
- Murias, M.; Webb, S.J.; Greenson, J.; Dawson, G. Resting State Cortical Connectivity Reflected in EEG Coherence in Individuals with Autism. Biol. Psychiatry 2007, 62, 270–273.
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267.
- Nelson, S.B.; Valakh, V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 2015, 87, 684–698.
- Trakoshis, S.; Martínez-Cañada, P.; Rocchi, F.; Canella, C.; You, W.; Chakrabarti, B.; Ruigrok, A.N.; Bullmore, E.T.; Suckling, J.; Markicevic, M.; et al. Intrinsic excitation-inhibition imbalance affects medial prefrontal cortex differently in autistic men versus women. eLife 2020, 9, e55684.
- Ecellot, G.; Echerubini, E. GABAergic Signaling as Therapeutic Target for Autism Spectrum Disorders. Front. Pediatr. 2014, 2, 70.
- Hussman, J. Suppressed GABAergic Inhibition as a Common Factor in Suspected Etiologies of Autism. J. Autism Dev. Disord. 2001, 31, 247–248.
- Lund, J.S.; Angelucci, A.; Bressloff, P.C. Anatomical Substrates for Functional Columns in Macaque Monkey Primary Visual Cortex. Cereb. Cortex 2003, 13, 15–24.
- Sohal, V.; Zhang, F.; Yizhar, O.; Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009, 459, 698–702.
- Keil, A.; Muller, M.M.; Ray, W.J.; Gruber, T.; Elbert, T. Human Gamma Band Activity and Perception of a Gestalt. J. Neurosci. 1999, 19, 7152–7161.
- Tiitinen, H.T.; Sinkkonen, J.; Reinikainen, K.; Alho, K.; Lavikainen, J.; Naatanen, R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature 1993, 364, 59–60.
- Gobbelé, R.; Waberski, T.D.; Schmitz, S.; Sturm, W.; Buchner, H. Spatial direction of attention enhances right hemispheric event-related gamma-band synchronization in humans. Neurosci. Lett. 2002, 327, 57–60.
- Fries, P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annu. Rev. Neurosci. 2009, 32, 209–224.
- Roux, F.; Uhlhaas, P.J. Working memory and neural oscillations: Alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn. Sci. 2014, 18, 16–25.
- Kucewicz, M.; Berry, B.M.; Kremen, V.; Brinkmann, B.; Sperling, M.R.; Jobst, B.C.; Gross, R.E.; Lega, B.; Sheth, S.A.; Stein, J.M.; et al. Dissecting gamma frequency activity during human memory processing. Brain 2017, 140, 1337–1350.
- Rojas, D.C.; Wilson, L.B. γ-band abnormalities as markers of autism spectrum disorders. Biomark. Med. 2014, 8, 353–368.
- Rojas, D.C.; Teale, P.D.; Maharajh, K.; Kronberg, E.; Youngpeter, K.; Wilson, L.B.; Wallace, A.; Hepburn, S. Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Mol. Autism 2011, 2, 11.
- Maxwell, C.R.; Villalobos, M.E.; Schultz, R.T.; Herpertz-Dahlmann, B.; Konrad, K.; Kohls, G. Atypical Laterality of Resting Gamma Oscillations in Autism Spectrum Disorders. J. Autism Dev. Disord. 2013, 45, 292–297.
- Courchesne, E. Abnormal early brain development in autism. Mol. Psychiatry 2002, 7 (Suppl. 2), S21–S23.
- Tang, G.; Gudsnuk, K.; Kuo, S.-H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron 2014, 83, 1131–1143.
- Rogers, S.J.; Wehner, E.A.; Hagerman, R. The Behavioral Phenotype in Fragile X: Symptoms of Autism in Very Young Children with Fragile X Syndrome, Idiopathic Autism, and Other Developmental Disorders. J. Dev. Behav. Pediatr. 2001, 22, 409–417.
- Fatemi, S.H.; Folsom, T.D.; Kneeland, R.E.; Liesch, S.B. Metabotropic Glutamate Receptor 5 Upregulation in Children with Autism is Associated with Underexpression of Both Fragile X Mental Retardation Protein and GABAA Receptor Beta 3 in Adults with Autism. Anat. Rec. 2011, 294, 1635–1645.
- Liu, S.; Zhou, L.; Yuan, H.; Vieira, M.; Sanz-Clemente, A.; Badger, J.D.; Lu, W.; Traynelis, S.F.; Roche, K.W. A Rare Variant Identified Within the GluN2B C-Terminus in a Patient with Autism Affects NMDA Receptor Surface Expression and Spine Density. J. Neurosci. 2017, 37, 4093–4102.
- Gandal, M.J.; Anderson, R.L.; Billingslea, E.N.; Carlson, G.C.; Roberts, T.P.L.; Siegel, S.J. Mice with reduced NMDA receptor expression: More consistent with autism than schizophrenia? Genes Brain Behav. 2012, 11, 740–750.
- D’Angelo, E. The cerebellum gets social. Science 2019, 363, 229.
- D’Angelo, E.; Casali, S. Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Front. Neural Circuits 2013, 6, 116.
- Schmahmann, J.D.; Caplan, D. Cognition, emotion and the cerebellum. Brain 2006, 129, 290–292.
- Wolf, U.; Rapoport, M.J.; Schweizer, T.A. Evaluating the Affective Component of the Cerebellar Cognitive Affective Syndrome. J. Neuropsychiatry Clin. Neurosci. 2009, 21, 245–253.
- Van Essen, D.C.; Donahue, C.J.; Glasser, M.F. Development and Evolution of Cerebral and Cerebellar Cortex. Brain Behav. Evol. 2018, 91, 158–169.
- Pijpers, W.; Apps, R.; Pardoe, J.; Voogd, J.; Ruigrok, T. Precise Spatial Relationships between Mossy Fibers and Climbing Fibers in Rat Cerebellar Cortical Zones. J. Neurosci. 2006, 26, 12067–12080.
- Oberdick, J.; Sillitoe, R.V. Cerebellar Zones: History, Development, and Function. Cerebellum 2011, 10, 301–306.
- Prestori, F.; Mapelli, L.; D’Angelo, E. Diverse Neuron Properties and Complex Network Dynamics in the Cerebellar Cortical Inhibitory Circuit. Front. Mol. Neurosci. 2019, 12, 267.
- Eito, M. Error detection and representation in the olivo-cerebellar system. Front. Neural Circuits 2013, 7, 1.
- Bengtsson, F.; Jorntell, H. Specific Relationship between Excitatory Inputs and Climbing Fiber Receptive Fields in Deep Cerebellar Nuclear Neurons. PLoS ONE 2014, 9, e84616.
- Steuber, V.; Jaeger, D. Modeling the generation of output by the cerebellar nuclei. Neural Netw. 2013, 47, 112–119.
- Eccles, J.; Llinás, R.; Sasaki, K. The inhibitory interneurones within the cerebellar cortex. Exp. Brain Res. 1966, 1, 1–16.
- Eccles, J.; Llinás, R.; Sasak, K. Inhibitory systems in the cerebellar cortex. Proc. Aust. Assoc. Neurol. 1965, 3, 7–14.
- Cesana, E.; Pietrajtis, K.; Bidoret, C.; Isope, P.; D’Angelo, E.U.; Dieudonné, S.; Forti, L. Granule Cell Ascending Axon Excitatory Synapses onto Golgi Cells Implement a Potent Feedback Circuit in the Cerebellar Granular Layer. J. Neurosci. 2013, 33, 12430–12446.
- Locatelli, F.; Soda, T.; Montagna, I.; Tritto, S.; Botta, L.; Prestori, F.; D’Angelo, E. Calcium Channel-Dependent Induction of Long-Term Synaptic Plasticity at Excitatory Golgi Cell Synapses of Cerebellum. J. Neurosci. 2021, 41, 3307–3319.
- Dieudonné, S. Submillisecond kinetics and low efficacy of parallel fibre-Golgi cell synaptic currents in the rat cerebellum. J. Physiol. 1998, 510 Pt 3, 845–866.
- Vos, B.P.; Volny-Luraghi, A.; De Schutter, E. Cerebellar Golgi cells in the rat: Receptive fields and timing of responses to facial stimulation. Eur. J. Neurosci. 1999, 11, 2621–2634.
- Eccles, J.; Llinás, R.; Sasaki, K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp. Brain Res. 1966, 1, 82–101.
- Evarts, E.V.; Thach, W.T. Motor Mechanisms of the CNS: Cerebrocerebellar Interrelations. Annu. Rev. Physiol. 1969, 31, 451–498.
- Schmahmann, J.D.; Pandya, D.N. The Cerebrocerebellar System. Int. Rev. Neurobiol. 1997, 41, 31–60.
- O’Reilly, J.X.; Beckmann, C.F.; Tomassini, V.; Ramnani, N.; Johansen-Berg, H. Distinct and Overlapping Functional Zones in the Cerebellum Defined by Resting State Functional Connectivity. Cereb. Cortex 2009, 20, 953–965.
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage 2009, 44, 489–501.
- Strata, P. The Emotional Cerebellum. Cerebellum 2015, 14, 570–577.
- Leggio, M.; Olivito, G. Topography of the cerebellum in relation to social brain regions and emotions. Handb. Clin. Neurol. 2018, 154, 71–84.
- Jones, E. The Thalamus; Springer Science & Business Media: New York, NY, USA, 2007.
- Chan-Palay, V.; Palay, S.L.; Brown, J.T.; Van Itallie, C. Sagittal organization of olivocerebellar and reticulocerebellar projections: Autoradiographic studies with 35S-methionine. Exp. Brain Res. 1977, 30, 561–576.
- Aumann, T.D.; Rawson, J.A.; Finkelstein, D.; Horne, M.K. Projections from the lateral and interposed cerebellar nuclei to the thalamus of the rat: A light and electron microscopic study using single and double anterograde labelling. J. Comp. Neurol. 1994, 349, 165–181.
- Georgescu, I.; Popa, D.; Zagrean, L. The Anatomical and Functional Heterogeneity of the Mediodorsal Thalamus. Brain Sci. 2020, 10, 624.
- Yamamoto, T.; Yoshida, K.; Yoshikawa, H.; Kishimoto, Y.; Oka, H. The medial dorsal nucleus is one of the thalamic relays of the cerebellocerebral responses to the frontal association cortex in the monkey: Horseradish peroxidase and fluorescent dye double staining study. Brain Res. 1992, 579, 315–320.
- Schmahmann, J. From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Hum. Brain Mapp. 1996, 4, 74–198.
- Palesi, F.; Ferrante, M.; Gaviraghi, M.; Misiti, A.; Savini, G.; Lascialfari, A.; D’Angelo, E.; Wheeler-Kingshott, C.A.M.G. Motor and higher-order functions topography of the human dentate nuclei identified with tractography and clustering methods. Hum. Brain Mapp. 2021, 42, 4348–4361.
- Palesi, F.; De Rinaldis, A.; Castellazzi, G.; Calamante, F.; Muhlert, N.; Chard, D.; Tournier, J.D.; Magenes, G.; D’Angelo, E.; Wheeler-Kingshott, C.A.G. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: Implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci. Rep. 2017, 7, 12841.
- Snider, R.S.; Maiti, A. Cerebellar contributions to the papez circuit. J. Neurosci. Res. 1976, 2, 133–146.
- Anand, B.K.; Malhotra, C.L.; Singh, B.; Dua, S. Cerebellar Projections to Limbic System. J. Neurophysiol. 1959, 22, 451–457.
- Bohne, P.; Schwarz, M.K.; Herlitze, S.; Mark, M.D. A New Projection from the Deep Cerebellar Nuclei to the Hippocampus via the Ventrolateral and Laterodorsal Thalamus in Mice. Front. Neural Circuits 2019, 13, 51.
- Colombel, C.; Lalonde, R.; Caston, J. The effects of unilateral removal of the cerebellar hemispheres on spatial learning and memory in rats. Brain Res. 2004, 1004, 108–115.
- Burguière, E.; Arabo, A.; Jarlier, F.; De Zeeuw, C.I.; Rondi-Reig, L. Role of the Cerebellar Cortex in Conditioned Goal-Directed Behavior. J. Neurosci. 2010, 30, 13265–13271.
- Barbas, H.; Blatt, G.J. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 1995, 5, 511–533.
- Hoover, W.B.; Vertes, R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007, 212, 149–179.
- Habas, C. Research note: A resting-state, cerebello-amygdaloid intrinsically connected network. Cerebellum Ataxias 2018, 5, 4.
- Sang, L.; Qin, W.; Liu, Y.; Han, W.; Zhang, Y.; Jiang, T.; Yu, C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage 2012, 61, 1213–1225.
- Heath, R.G.; Harper, J.W. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: Evoked potential and histological studies in monkeys and cats. Exp. Neurol. 1974, 45, 268–287.
- Morris, J.S.; Frith, C.; Perrett, D.I.; Rowland, D.; Young, A.W.; Calder, A.J.; Dolan, R. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 1996, 383, 812–815.
- Ernst, T.M.; Brol, A.E.; Gratz, M.; Ritter, C.; Bingel, U.; Schlamann, M.; Maderwald, S.; Quick, H.H.; Merz, C.J.; Timmann, D. The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. eLife 2019, 8, e46831.
- Vilensky, J.A.; Van Hoesen, G.W. Corticopontine projections from the cingulate cortex in the rhesus monkey. Brain Res. 1981, 205, 391–395.
- Krienen, F.M.; Buckner, R.L. Segregated Fronto-Cerebellar Circuits Revealed by Intrinsic Functional Connectivity. Cereb. Cortex 2009, 19, 2485–2497.
- Rogers, T.D.; Dickson, P.E.; Heck, D.H.; Goldowitz, D.; Mittleman, G.; Blaha, C.D. Connecting the dots of the cerebro-cerebellar role in cognitive function: Neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse 2011, 65, 1204–1212.
- Mittleman, G.; Goldowitz, D.; Heck, D.H.; Blaha, C.D. Cerebellar modulation of frontal cortex dopamine efflux in mice: Relevance to autism and schizophrenia. Synapse 2008, 62, 544–550.
- Watson, T.; Becker, N.; Apps, R.; Jones, M. Back to front: Cerebellar connections and interactions with the prefrontal cortex. Front. Syst. Neurosci. 2014, 8, 4.
- Forster, G.L.; Blaha, C.D. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of ace-tylcholine and glutamate receptors in the midbrain and pons of the rat. Eur. J. Neurosci. 2003, 17, 751–762.
- Garcia-Rill, E.; Skinner, R.; Miyazato, H.; Homma, Y. Pedunculopontine stimulation induces prolonged activation of pontine reticular neurons. Neuroscience 2001, 104, 455–465.
- Perciavalle, V.; Berretta, S.; Raffaele, R. Projections from the intracerebellar nuclei to the ventral midbrain tegmentum in the rat. Neuroscience 1989, 29, 109–119.
- Schwarz, C.; Schmitz, Y. Projection from the cerebellar lateral nucleus to precerebellar nuclei in the mossy fiber pathway is glutamatergic: A study combining anterograde tracing with immunogold labeling in the rat. J. Comp. Neurol. 1997, 381, 320–334.
- Pinto, A.; Jankowski, M.; Sesack, S.R. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: Ultrastructural characteristics and spatial relationships with dopamine afferents. J. Comp. Neurol. 2003, 459, 142–155.
- Del Arco, A.; Mora, F. Glutamate-dopamine in vivo interaction in the prefrontal cortex modulates the release of dopamine and acetylcholine in the nucleus accumbens of the awake rat. J. Neural Transm. 2005, 112, 97–109.
- Carta, I.; Chen, C.H.; Schott, A.L.; Dorizan, S.; Khodakhah, K. Cerebellar modulation of the reward circuitry and social behavior. Science 2019, 363, eaav0581.
- D’Mello, A.; Stoodley, C.J. Cerebro-cerebellar circuits in autism spectrum disorder. Front. Neurosci. 2015, 9, 408.
- Rogers, T.D.; Dickson, P.E.; McKimm, E.; Heck, D.H.; Goldowitz, D.; Blaha, C.D.; Mittleman, G. Reorganization of Circuits Underlying Cerebellar Modulation of Prefrontal Cortical Dopamine in Mouse Models of Autism Spectrum Disorder. Cerebellum 2013, 12, 547–556.
- Wang, S.S.-H.; Kloth, A.D.; Badura, A. The Cerebellum, Sensitive Periods, and Autism. Neuron 2014, 83, 518–532.
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum 2012, 11, 777–807.
- Reeber, S.L.; Otis, T.S.; Sillitoe, R.V. New roles for the cerebellum in health and disease. Front. Syst. Neurosci. 2013, 7, 83.
- Bauman, M.; Kemper, T.L. Histoanatomic observations of the brain in early infantile autism. Neurology 1985, 35, 866.
- Kemper, T.L.; Bauman, M.L. The Contribution of Neuropathologic Studies to the Understanding of Autism. Neurol. Clin. 1993, 11, 175–187.
- Bailey, A.; Luthert, P.; Dean, A.; Harding, B.; Janota, I.; Montgomery, M.; Rutter, M.L.; Lantos, P. A clinicopathological study of autism. Brain 1998, 121 Pt 5, 889–905.
- Whitney, E.R.; Kemper, T.L.; Bauman, M.L.; Rosene, D.; Blatt, G.J. Cerebellar Purkinje Cells are Reduced in a Subpopulation of Autistic Brains: A Stereological Experiment Using Calbindin-D28k. Cerebellum 2008, 7, 406–416.
- Skefos, J.; Cummings, C.; Enzer, K.; Holiday, J.; Weed, K.; Levy, E.; Yuce, T.; Kemper, T.; Bauman, M. Regional Alterations in Purkinje Cell Density in Patients with Autism. PLoS ONE 2014, 9, e81255.
- Palmen, S.J.M.C.; van Engeland, H.; Hof, P.R.; Schmitz, C. Neuropathological findings in autism. Brain 2004, 127, 2572–2583.
- Fatemi, S.H.; Halt, A.R.; Realmuto, G.; Earle, J.; Kist, D.A.; Thuras, P.; Merz, A. Purkinje Cell Size Is Reduced in Cerebellum of Patients with Autism. Cell. Mol. Neurobiol. 2002, 22, 171–175.
- Bauman, M.; Kemper, T. (Eds.) Structural brain anatomy in autism: What is evidence? In The Neurobiology of Autism; JHU Press: Baltimore, MD, USA, 2005; pp. 119–145.
- Whitney, E.R.; Kemper, T.L.; Rosene, D.L.; Bauman, M.L.; Blatt, G.J. Density of cerebellar basket and stellate cells in autism: Evidence for a late developmental loss of Purkinje cells. J. Neurosci. Res. 2009, 87, 2245–2254.
- Crepel, F.; Mariani, J. Multiple innervation of purkinje cells by climbing fibers in the cerebellum of the weaver mutant mouse. J. Neurobiol. 1976, 7, 579–582.
- Puro, D.G.; Woodward, D.J. The climbing fiber system in the Weaver mutant. Brain Res. 1977, 129, 141–146.
- Mariani, J. Extent of multiple innervation of purkinje cells by climbing fibers in the olivocerebellar system of weaver, reeler, and staggerer mutant mice. J. Neurobiol. 1982, 13, 119–126.
- Cajal, S. Histology of the Nervous System of Man and Vertebrates; Oxford University Press: New York, NY, USA, 1995; pp. 1909–1910.
- Blatt, G.J. The Neuropathology of Autism. Scientifica 2012, 2012, 703675.
- Kemper, T.L.; Bauman, M.L. Neuropathology of infantile autism. Mol. Psychiatry 2002, 7 (Suppl. 2), S12–S13.
- Hampson, D.R.; Blatt, G.J. Autism spectrum disorders and neuropathology of the cerebellum. Front. Neurosci. 2015, 9, 420.
- Courchesne, E.; Townsend, J.; Akshoomoff, N.A.; Saitoh, O.; Yeung-Courchesne, R.; Lincoln, A.J.; James, H.E.; Haas, R.H.; Schreibman, L.; Lau, L. Impairment in shifting attention in autistic and cerebellar patients. Behav. Neurosci. 1994, 108, 848–865.
- Courchesne, E.; Yeung-Courchesne, R.; Hesselink, J.; Jernigan, T. Hypoplasia of Cerebellar Vermal Lobules VI and VII in Autism. N. Engl. J. Med. 1988, 318, 1349–1354.
- Murakami, J.W.; Courchesne, E.; Press, G.A.; Yeung-Courchesne, R.; Hesselink, J.R. Reduced Cerebellar Hemisphere Size and Its Relationship to Vermal Hypoplasia in Autism. Arch. Neurol. 1989, 46, 689–694.
- Kaufmann, W.E.; Cooper, K.L.; Mostofsky, S.H.; Capone, G.T.; Kates, W.R.; Newschaffer, C.J.; Bukelis, I.; Stump, M.H.; Jann, A.E.; Lanham, D.C. Specificity of Cerebellar Vermian Abnormalities in Autism: A Quantitative Magnetic Resonance Imaging Study. J. Child Neurol. 2003, 18, 463–470.
- Scott, J.A.; Schumann, C.M.; Goodlin-Jones, B.L.; Amaral, D.G. A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Res. 2009, 2, 246–257.
- Piven, J.; Saliba, K.; Bailey, J.; Arndt, S. An MRI study of autism: The cerebellum revisited. Neurology 1997, 49, 546–551.
- Sparks, B.F.; Friedman, S.D.; Shaw, D.W.; Aylward, E.H.; Echelard, D.; Artru, A.A.; Maravilla, K.R.; Giedd, J.N.; Munson, J.; Dawson, G.; et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002, 59, 184–192.
- Courchesne, E.; Pierce, K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. Int. J. Dev. Neurosci. 2005, 23, 153–170.
- Courchesne, E.; Karns, C.M.; Davis, H.R.; Ziccardi, R.; Carper, R.A.; Tigue, Z.D.; Chisum, H.J.; Moses, P.; Pierce, K.; Lord, C.; et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology 2001, 57, 245–254.
- Khan, A.J.; Nair, A.; Keown, C.L.; Datko, M.C.; Lincoln, A.J.; Müller, R.-A. Cerebro-cerebellar Resting-State Functional Connectivity in Children and Adolescents with Autism Spectrum Disorder. Biol. Psychiatry 2015, 78, 625–634.
- Verly, M.; Verhoeven, J.; Zink, I.; Mantini, D.; Peeters, R.; Deprez, S.; Emsell, L.; Boets, B.; Noens, I.; Steyaert, J.; et al. Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. NeuroImage Clin. 2014, 4, 374–382.
- Lee, J.M.; Kyeong, S.; Kim, E.; Cheon, K.-A. Abnormalities of Inter- and Intra-Hemispheric Functional Connectivity in Autism Spectrum Disorders: A Study Using the Autism Brain Imaging Data Exchange Database. Front. Neurosci. 2016, 10, 191.
- Monk, C.S.; Peltier, S.J.; Wiggins, J.L.; Weng, S.-J.; Carrasco, M.; Risi, S.; Lord, C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage 2009, 47, 764–772.
- Uddin, L.Q.; Supekar, K.; Menon, V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013, 7, 458.
- Hahamy, A.; Behrmann, M.; Malach, R. The idiosyncratic brain: Distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat. Neurosci. 2015, 18, 302–309.
- Nunes, A.S.; Peatfield, N.; Vakorin, V.; Doesburg, S.M. Idiosyncratic organization of cortical networks in autism spectrum disorder. NeuroImage 2019, 190, 182–190.
- Jack, A.; Morris, J.P. Neocerebellar contributions to social perception in adolescents with autism spectrum disorder. Dev. Cogn. Neurosci. 2014, 10, 77–92.
- Fatemi, S.H.; Stary, J.M.; Halt, A.R.; Realmuto, G.R. Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J. Autism Dev. Disord. 2001, 31, 529–535.
- Chugani, D.C.; Muzik, O.; Rothermel, R.; Behen, M.E.; Chakraborty, P.K.; Mangner, T.J.; Chugani, H.T. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann. Neurol. 1997, 42, 666–669.
- Chugani, D.C. Role of altered brain serotonin mechanisms in autism. Mol. Psychiatry 2002, 7 (Suppl. 2), S16–S17.
- Vichier-Guerre, C.; Parker, M.; Pomerantz, Y.; Finnell, R.; Cabrera, R.M. Impact of selective serotonin reuptake inhibitors on neural crest stem cell formation. Toxicol. Lett. 2017, 281, 20–25.
- Fricker, A.D.; Rios, C.; Devi, L.A.; Gomes, I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Mol. Brain Res. 2005, 138, 228–235.
- Khozhai, L.I.; Otellin, V.A. Synaptogenesis in the dorsal raphe nucleus of rat medulla oblongata in serotonin deficiency. Morfologiia 2012, 142, 20–24.
- Fatemi, S.; Halt, A.R.; Stary, J.M.; Kanodia, R.; Schulz, S.; Realmuto, G.R. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol. Psychiatry 2002, 52, 805–810.
- Yip, J.; Soghomonian, J.-J.; Blatt, G.J. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: Pathophysiological implications. Acta Neuropathol. 2007, 113, 559–568.
- Yip, J.; Soghomonian, J.-J.; Blatt, G.J. IncreasedGAD67 mRNA expression in cerebellar interneurons in autism: Implications for Purkinje cell dysfunction. J. Neurosci. Res. 2007, 86, 525–530.
- Llinas, R.; Leznik, E.; Makarenko, V. The Olivo-Cerebellar Circuit as a Universal Motor Control System. IEEE J. Ocean. Eng. 2004, 29, 631–639.
- Yip, J.; Soghomonian, J.J.; Blatt, G.J. Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: An in situ hybridization study. Autism Res. 2009, 2, 50–59.
- Hegarty, J.P.; Weber, D.J.; Cirstea, C.M.; Beversdorf, D.Q. Cerebro-Cerebellar Functional Connectivity is Associated with Cerebellar Excitation–Inhibition Balance in Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 3460–3473.
- Faissner, A.; Reinhard, J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia 2015, 63, 1330–1349.
- Dityatev, A.; Schachner, M.; Sonderegger, P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 2010, 11, 735–746.
- Ahmad, S.F.; Nadeem, A.; Ansari, M.A.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Attia, S.M. Upregulation of IL-9 and JAK-STAT signaling pathway in children with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 472–480.
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90.
- Meltzer, A.; Van De Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 284–298.
- Siniscalco, D.; Schultz, S.; Brigida, A.L.; Antonucci, N. Inflammation and Neuro-Immune Dysregulations in Autism Spectrum Disorders. Pharmaceuticals 2018, 11, 56.
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 2016, 6, e844.
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81.
- Wei, H.; Zou, H.; Sheikh, A.M.; Malik, M.; Dobkin, C.; Brown, W.T.; Li, X. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J. Neuroinflamm. 2011, 8, 52.
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.-M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116.
- Lucchina, L.; Depino, A.M. Altered Peripheral and Central Inflammatory Responses in a Mouse Model of Autism. Autism Res. 2014, 7, 273–289.
- Goines, P.; Haapanen, L.; Boyce, R.; Duncanson, P.; Braunschweig, D.; Delwiche, L.; Hansen, R.; Hertz-Picciotto, I.; Ashwood, P.; Van de Water, J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav. Immun. 2011, 25, 514–523.
- Wills, S.; Cabanlit, M.; Bennett, J.; Ashwood, P.; Amaral, D.G.; Van de Water, J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav. Immun. 2009, 23, 64–74.
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.N.; Van de Water, J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2011, 232, 196–199.
- Heuer, L.; Ashwood, P.; Schauer, J.; Goines, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Croen, L.A.; Pessah, I.N.; Van De Water, J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008, 1, 275–283.
- Wills, S.; Rossi, C.C.; Bennett, J.; Martinez-Cerdeño, V.; Ashwood, P.; Amaral, D.G.; Van de Water, J. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Mol. Autism 2011, 2, 5.
- Bolivar, V.J.; Walters, S.R.; Phoenix, J.L. Assessing autism-like behavior in mice: Variations in social interactions among inbred strains. Behav. Brain Res. 2007, 176, 21–26.
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like behavioral phenotypes in BTBR T + tf/J mice. Genes Brain Behav. 2008, 7, 152–163.
- Heo, Y.; Zhang, Y.; Gao, D.; Miller, V.M.; Lawrence, D.A. Aberrant Immune Responses in a Mouse with Behavioral Disorders. PLoS ONE 2011, 6, e20912.
- Bakheet, S.A.; Alzahrani, M.Z.; Nadeem, A.; Ansari, M.A.; Zoheir, K.; Attia, S.M.; Al-Ayadhi, L.Y.; Ahmad, S.F. Resveratrol treatment attenuates chemokine receptor expression in the BTBR T + tf/J mouse model of autism. Mol. Cell. Neurosci. 2016, 77, 1–10.
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alzahrani, M.Z.; Alshammari, M.A.; Alanazi, W.A.; Alasmari, A.F.; Attia, S.M. Resveratrol attenuates pro-inflammatory cytokines and activation of JAK1-STAT3 in BTBR T + Itpr3 tf /J autistic mice. Eur. J. Pharmacol. 2018, 829, 70–78.
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Al-Ayadhi, L.Y.; Attia, S.M. Toll-like receptors, NF-κB, and IL-27 mediate adenosine A2A receptor signaling in BTBR T + Itpr3 tf /J mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 184–191.
- Xiao, R.; Zhong, H.; Li, X.; Ma, Y.; Zhang, R.; Wang, L.; Zang, Z.; Fan, X. Abnormal Cerebellar Development Is Involved in Dystonia-Like Behaviors and Motor Dysfunction of Autistic BTBR Mice. Front. Cell Dev. Biol. 2020, 8, 231.
This entry is offline, you can click here to edit this entry!
