Zeaxanthin and lutein are structural isomers with zeaxanthin possessing a slightly longer system of conjugated double bonds (11) than lutein. Carotenoids also play key roles in light-independent processes, e.g., as gene regulators of human immune function. Carotenoid-derived vitamin A has a well-documented immunoregulatory role and a similar role is emerging for xanthophylls. Xanthophylls may be especially important in opposing non-resolving inflammation that can trigger a plethora of associated inflammatory diseases, disorders, and dysfunctions. Additionally, lutein and zeaxanthin are emerging as candidates for protecting cognitive function across the human lifespan, including attention, memory, learning, and executive functions.
- antioxidant
- carotenoid
- inflammation
- lutein
- photosynthesis
- retina
- ROS
- zeaxanthin
1. Introduction
Carotenoids in a Nutshell
2. Xanthophylls in High-Stress Contexts
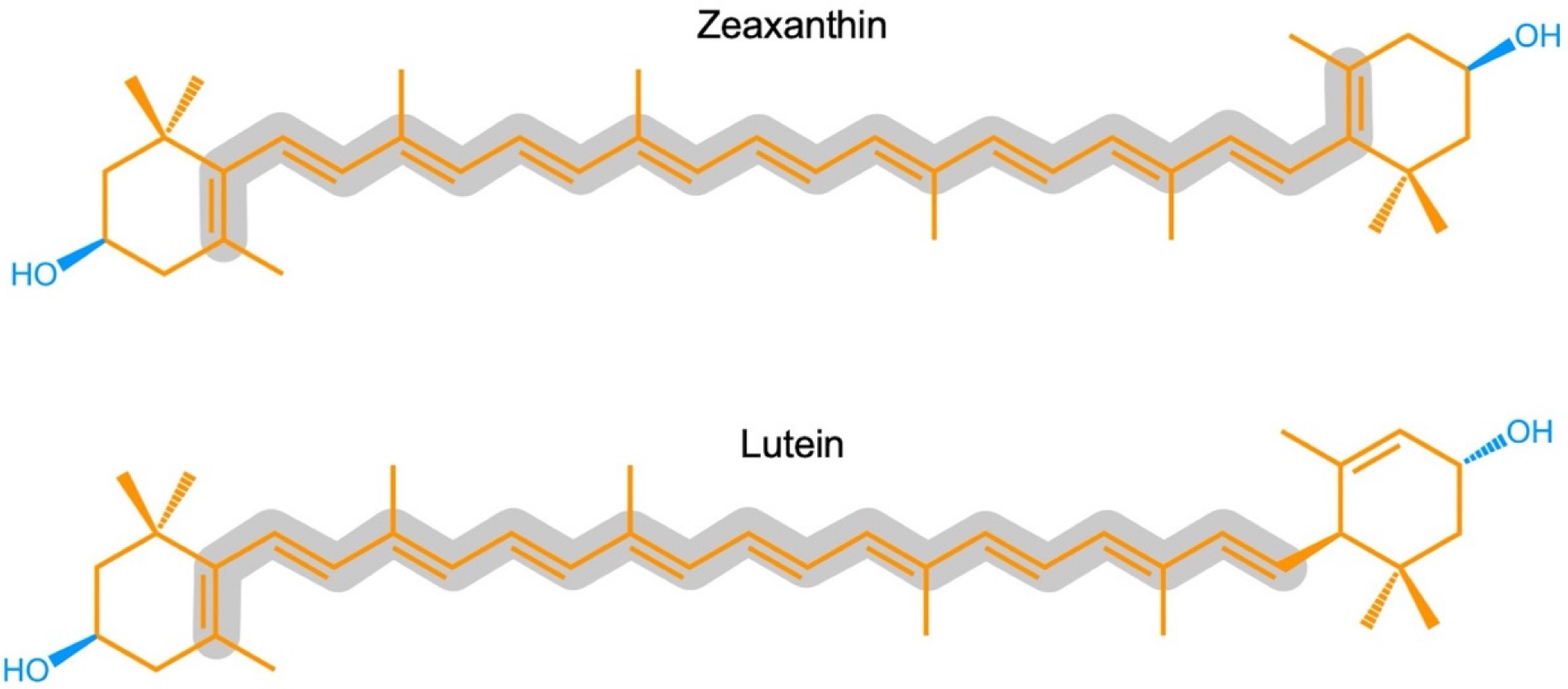
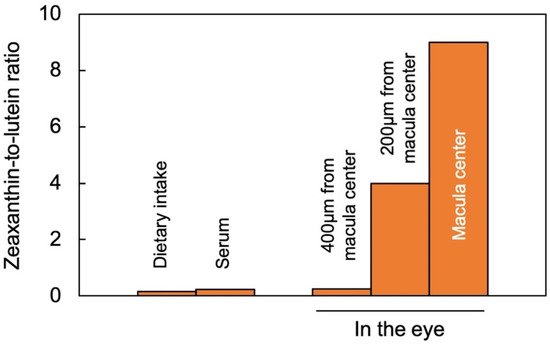
2.1. Zeaxanthin and Lutein in the Human Eye/Retina
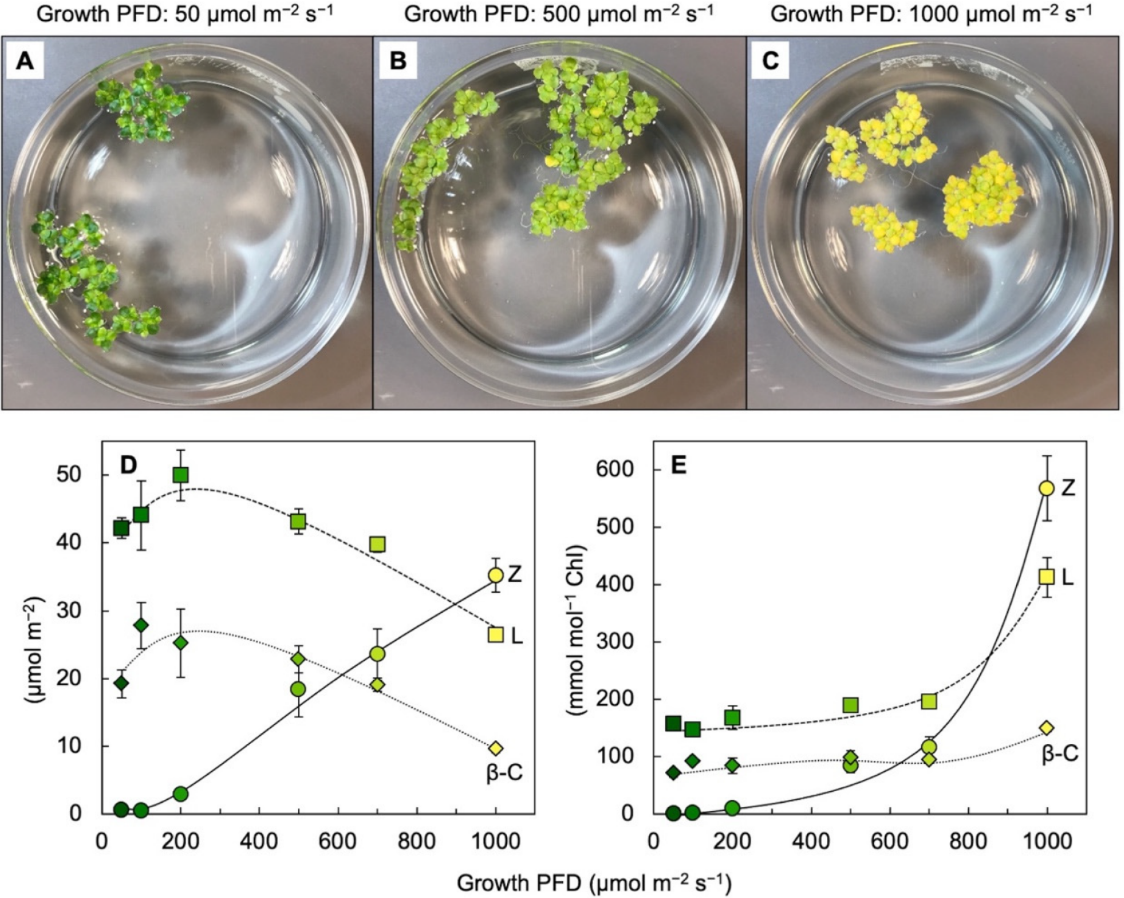
2.2. Zeaxanthin and Lutein in Leaves
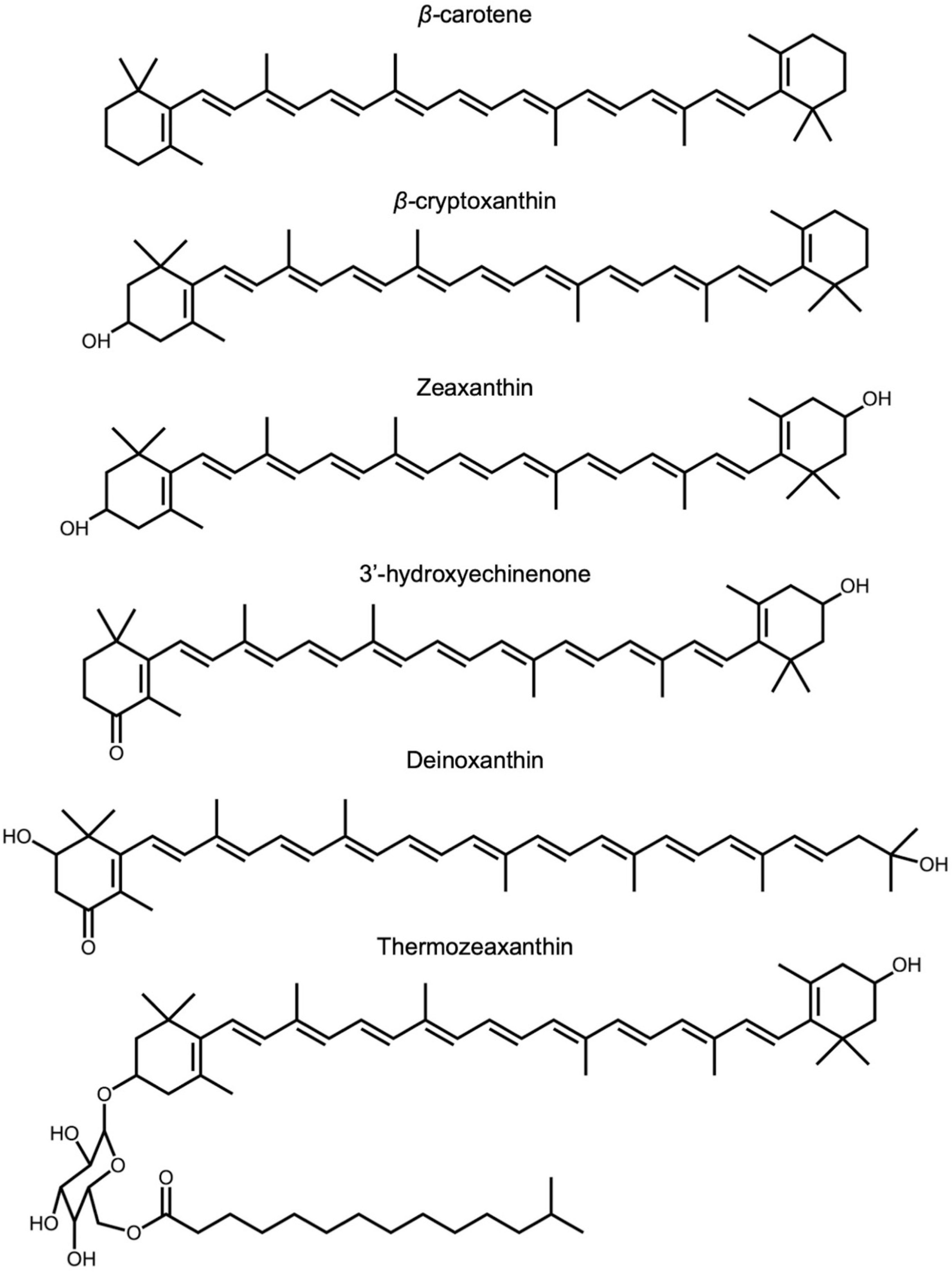
2.3. Zeaxanthin and Related Xanthophylls in Extremophiles
3. Association with Proteins and/or Phospholipid Bilayers
3.1. Association of Lutein and Zeaxanthin with Proteins—Selected Examples across Taxa
3.2. Lutein and Zeaxanthin Localization within the Phospholipid Bilayer of Biological Membranes—Selected Examples across Taxa
This entry is adapted from the peer-reviewed paper 10.3390/photochem2020022
References
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004.
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Natural Functions; Birkhäuser Verlag: Basel, Switzerland, 2008; Volume 4.
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Nutrition and Health; Birkhäuser Verlag: Basel, Switzerland, 2009; Volume 5.
- Moran, N.A.; Jarvik, T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 2010, 328, 624–627.
- Nishino, A.; Maoka, T.; Yasui, H. Preventive effects of β-cryptoxanthin, a potent antioxidant and provitamin A carotenoid, on lifestyle-related diseases—A central focus on its effects on non-alcoholic fatty liver disease (NAFLD). Antioxidants 2022, 11, 43.
- Hofmann, E.; Hiller, R.G.; Welte, W.; Diederichs, K. Light harvesting by carotenoids: Peridinin-chlorophyll-protein (PCP) from Amphidinium carterae structural relation to proteins with globin fold. Z. Krist. Suppl. 1997, 11, 58.
- Nagao, R.; Yokono, M.; Teshigahara, A.; Akimoto, S.; Tomo, T. Light-harvesting ability of the fucoxanthin chlorophyll a/c-binding protein associated with photosystem II from the diatom Chaetoceros gracilis as revealed by picosecond time-resolved fluorescence spectroscopy. J. Phys. Chem. B 2014, 118, 5093–5100.
- Polívka, T.; Hiller, R.G.; Frank, H.A. Spectroscopy of the peridinin–chlorophyll-a protein: Insight into light-harvesting strategy of marine algae. Arch. Biochem. Biophys. 2007, 458, 111–120.
- Wang, W.; Qin, X.; Sang, M.; Chen, D.; Wang, K.; Lin, R.; Lu, C.; Shen, J.R.; Kuang, T. Spectral and functional studies on siphonaxanthin-type light-harvesting complex of photosystem II from Bryopsis corticulans. Photosynth. Res. 2013, 117, 267–279.
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825.
- Schalch, W.; Landrum, J.T.; Bone, R.A. The eye. In Carotenoids. Nutrition and Health; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; Volume 5, pp. 301–334.
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832.
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261.
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607.
- Stringham, J.M.; Johnson, E.J.; Hammond, B.R. Lutein across the lifespan: From childhood cognitive performance to the aging eye and brain. Curr. Dev. Nutr. 2019, 3, nzz066.
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2013, 79, 597–606.
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.-L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278.
- Vershinin, A. Biological functions of carotenoids-Diversity and evolution. BioFactors 1999, 10, 99–104.
- Polívka, T.; Frank, H.A. Spectroscopic investigation of carotenoids involved in non-photochemical fluorescence quenching. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 203–227.
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201.
- Johnson, E.J.; Neuringer, M.; Russell, R.M.; Schalch, W.; Snodderly, D.M. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investig. Opthalmol. Vis. Sci. 2005, 46, 692.
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358.
- Widomska, J.; Zareba, M.; Subczynski, W. Can xanthophyll-membrane interactions explain their selective presence in the retina and brain? Foods 2016, 5, 7.
- Adams, W.W., III; Demmig-Adams, B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 1992, 186, 390–398.
- Adams, W.W., III; Demmig-Adams, B. Chlorophyll fluorescence as a tool to monitor plant response to the environment. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 583–604.
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62.
- Hager, A. The reversible, light-induced conversions of xanthophylls in the chloroplast. In Pigments in Plants; Czygan, F.-C., Ed.; Fischer: Stuttgart, Germany, 1980; pp. 57–79.
- Yamamoto, H.Y. A random walk to and through the xanthophyll cycle. In Photoprotection, Photoinhibition, Gene Regulation, and Environment. Advances in Photosynthesis and Respiration; Demmig-Adams, B., Adams, W.W., III, Mattoo, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 21, pp. 1–10.
- Demmig-Adams, B.; López-Pozo, M.; Polutchko, S.K.; Fourounjian, P.; Stewart, J.J.; Zenir, M.C.; Adams, W.W., III. Growth and nutritional quality of Lemnaceae viewed comparatively in an ecological and evolutionary context. Plants 2022, 11, 145.
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020, 11, 480.
- Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Doherty Garcia, N.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Features of the duckweed Lemna that support rapid growth under extremes of light intensity. Cells 2021, 10, 1481.
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767.
- Havaux, M.; García-Plazaola, J.I. Beyond non-photochemical fluorescence quenching: The overlapping antioxidant functions of zeaxanthin and tocopherols. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603.
- Michael, T.P.; Ernst, E.; Hartwick, N.; Chu, P.; Bryant, D.; Gilbert, S.; Ortleb, S.; Baggs, E.L.; Sree, K.S.; Appenroth, K.J.; et al. Genome and time-of-day transcriptome of Wolffia australiana link morphological minimization with gene loss and less growth control. Genome Res. 2021, 31, 225–238.
- Arunkumar, R.; Calvo, C.M.; Conrady, C.D.; Bernstein, P.S. What do we know about the macular pigment in AMD: The past, the present, and the future. Eye 2018, 32, 992–1004.
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206.
- Alcaíno, J.; Baeza, M.; Cifuentes, V. Carotenoid distribution in nature. In Carotenoids in Nature; Stange, C., Ed.; Springer: Cham, Switzerland, 2016; Volume 79, pp. 3–33.
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398.
- Nelis, H.J.; De Leenheer, A.P. Microbial sources of carotenoid pigments used in foods and feeds. J. Appl. Bacteriol. 1991, 70, 181–191.
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61.
- Ničková, K.; Štys, D. Carotenoid pigments in three Synechococcus strains. Algol. Stud. Hydrobiol. Suppl. 2003, 109, 421–428.
- Bemal, S.; Anil, A.C. Genetic and ecophysiological traits of Synechococcus strains isolated from coastal and open ocean waters of the Arabian Sea. FEMS Microbiol. Ecol. 2016, 92, fiw162.
- Wilson, A.; Muzzopappa, F.; Kirilovsky, D. Elucidation of the essential amino acids involved in the binding of the cyanobacterial orange carotenoid protein to the phycobilisome. Biochim. Biophys. Acta BBA-Bioenerg. 2022, 1863, 148504.
- Berry, A.; Janssens, D.; Hümbelin, M.; Jore, J.P.; Hoste, B.; Cleenwerck, I.; Vancanneyt, M.; Bretzel, W.; Mayer, A.F.; Lopez-Ulibarri, R.; et al. Paracoccus zeaxanthinifaciens sp. nov., a zeaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 2003, 53, 231–238.
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 103867.
- Ghedira, K.; Othman, H.; Saied, T.; Baccar, Z.M.; Hosni, F.; Hamzaoui, A.H.; Thamaraiselvi, K.; Abdelmelek, H.; Srairi-Abid, N.; Costa, M.C.; et al. Insights into ionizing-radiation-resistant bacteria S-layer proteins and nanobiotechnology for bioremediation of hazardous and radioactive waste. In Management of Hazardous Wastes; Saleh, H.E.-D.M., Rahman, R.O.A., Eds.; IntechOpen: London, UK, 2016.
- Adamec, F.; Farci, D.; Bína, D.; Litvín, R.; Khan, T.; Fuciman, M.; Piano, D.; Polívka, T. Photophysics of deinoxanthin, the keto-carotenoid bound to the main S-layer unit of Deinococcus radiodurans. Photochem. Photobiol. Sci. 2020, 19, 495–503.
- Asker, D.; Beppu, T.; Ueda, K. Unique diversity of carotenoid-producing bacteria isolated from Misasa, a radioactive site in Japan. Appl. Microbiol. Biotechnol. 2007, 77, 383–392.
- Farci, D.; Kereïche, S.; Pangeni, S.; Haniewicz, P.; Bodrenko, I.V.; Ceccarelli, M.; Winterhalter, M.; Piano, D. Structural analysis of the architecture and in situ localization of the main S-layer complex in Deinococcus radiodurans. Structure 2021, 29, 1279–1285.e3.
- Tian, B.; Xu, Z.; Sun, Z.; Lin, J.; Hua, Y. Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochim. Biophys. Acta BBA-Gen. Subj. 2007, 1770, 902–911.
- Gudkov, S.V.; Grinberg, M.A.; Sukhov, V.; Vodeneev, V. Effect of ionizing radiation on physiological and molecular processes in plants. J. Environ. Radioact. 2019, 202, 8–24.
- Hara, M.; Yuan, H.; Yang, Q.; Hoshino, T.; Yokoyama, A.; Miyake, J. Stabilization of liposomal membranes by thermozeaxanthins: Carotenoid-glucoside esters. Biochim. Biophys. Acta BBA-Biomembr. 1999, 1461, 147–154.
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790.
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N.; et al. Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front. Microbiol. 2014, 5, 100.
- Demmig-Adams, B.; Garab, G.; Adams, W.W., III. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2014; Volume 40.
- Skotnicová, P.; Staleva-Musto, H.; Kuznetsova, V.; Bína, D.; Konert, M.M.; Lu, S.; Polívka, T.; Sobotka, R. Plant LHC-like proteins show robust folding and static non-photochemical quenching. Nat. Commun. 2021, 12, 6890.
- Kirilovsky, D.; Kerfeld, C.A. Cyanobacterial photoprotection by the orange carotenoid protein. Nat. Plants 2016, 2, 16180.
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2020, 1865, 158617.
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 2004, 279, 49447–49454.
- Li, B.; Vachali, P.; Frederick, J.M.; Bernstein, P.S. Identification of StARD3 as a lutein-binding protein in the macula of the primate Retina. Biochemistry 2011, 50, 2541–2549.
- Rohmer, M.; Bouvier, P.; Ourisson, G. Molecular evolution of biomembranes: Structural equivalents and phylogenetic precursors of sterols. Proc. Natl. Acad. Sci. USA 1979, 76, 847–851.
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786.
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2005, 1740, 108–115.
- Makuch, K.; Hryc, J.; Markiewicz, M.; Pasenkiewicz-Gierula, M. Lutein and zeaxanthin in the lipid bilayer–Similarities and differences revealed by computational studies. Front. Mol. Biosci. 2021, 8, 768449.
- Widomska, J.; Subczynski, W.K. Why has nature chosen lutein and zeaxanthin to protect the retina? J. Clin. Exp. Ophthalmol. 2014, 5, 326.
- Widomska, J.; Gruszecki, W.I.; Subczynski, W.K. Factors differentiating the antioxidant activity of macular santhophylls in the human eye retina. Antioxidants 2021, 10, 601.
- Wisniewska, A.; Subczynski, W.K. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic. Biol. Med. 2006, 40, 1820–1826.
