High energy demand from the market due to the rapid increment of the human population worldwide has urged society to explore alternatives to replace non-renewable energy. Renewable diesel produced from biomass could be the next potential energy source for its high stability, long-term storage, and comparable performance with diesel fuels. In producing renewable diesel, the application of catalyst is essential, and the catalyst support is synthesized with the catalyst to enhance the reaction rate and catalytic properties. The application of the supported catalyst in increasing the selectivity and yield of renewable diesel is significant, in which the catalytic properties depend on the interaction between catalyst and catalyst support. The supported catalyst as a favorable substance to assist in enhancing renewable diesel yield could lead to a sustainable and greener future for the biofuel industry in Malaysia.
- catalyst support
- renewable diesel
- recyclability
- stability
- enhancement
1. Introduction
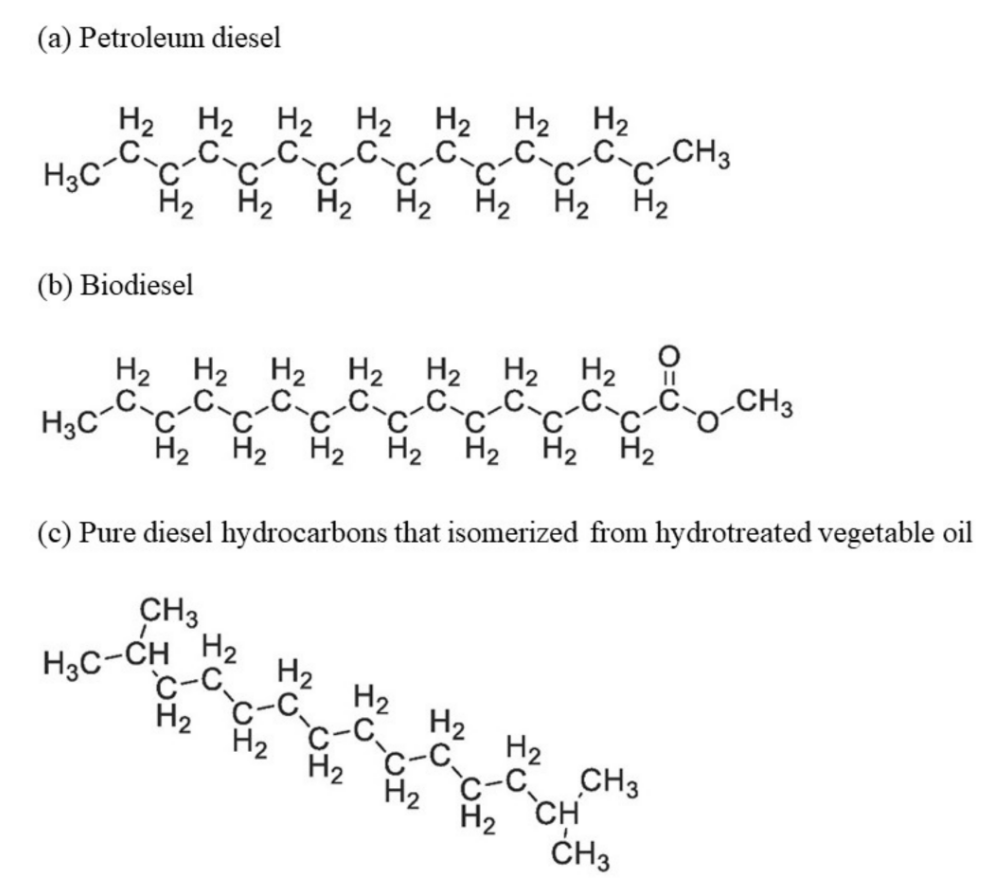
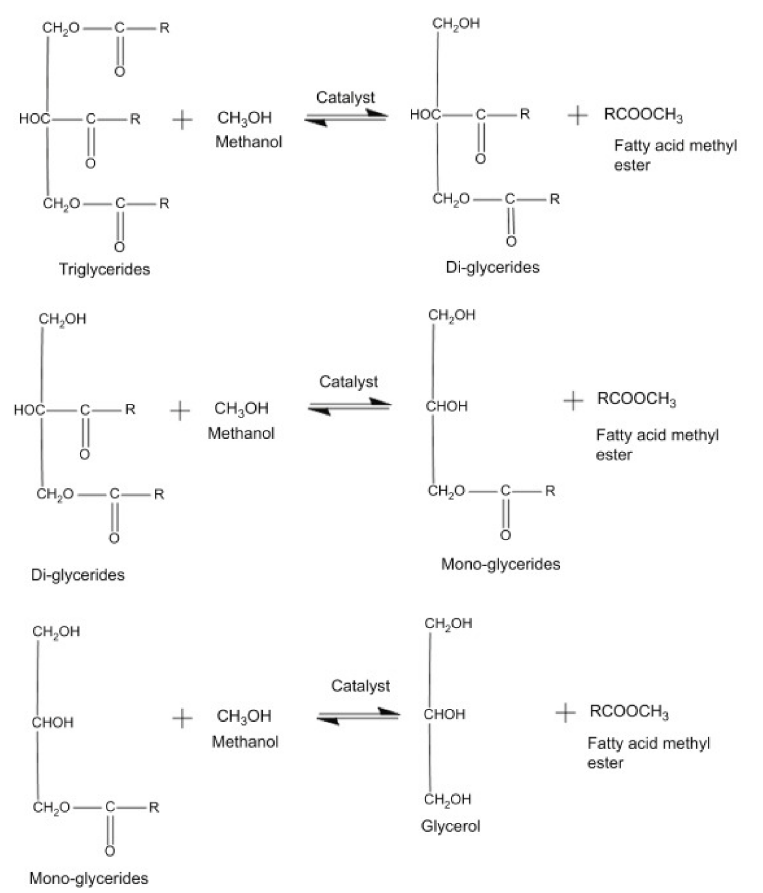
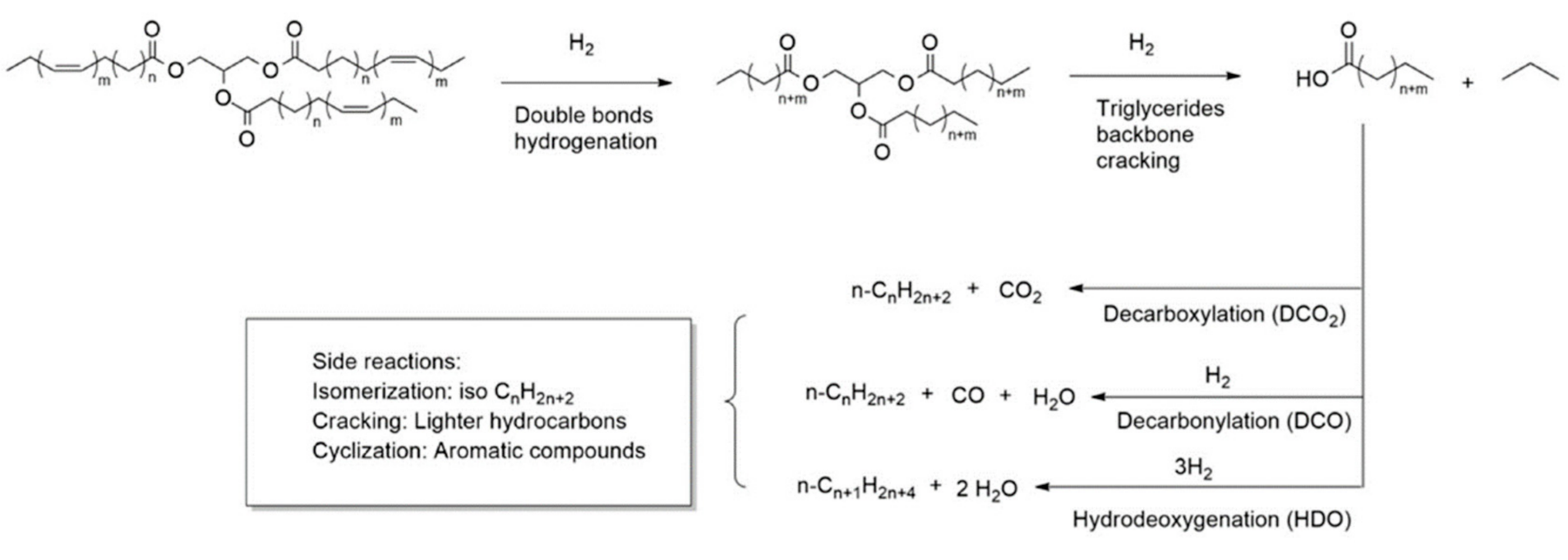
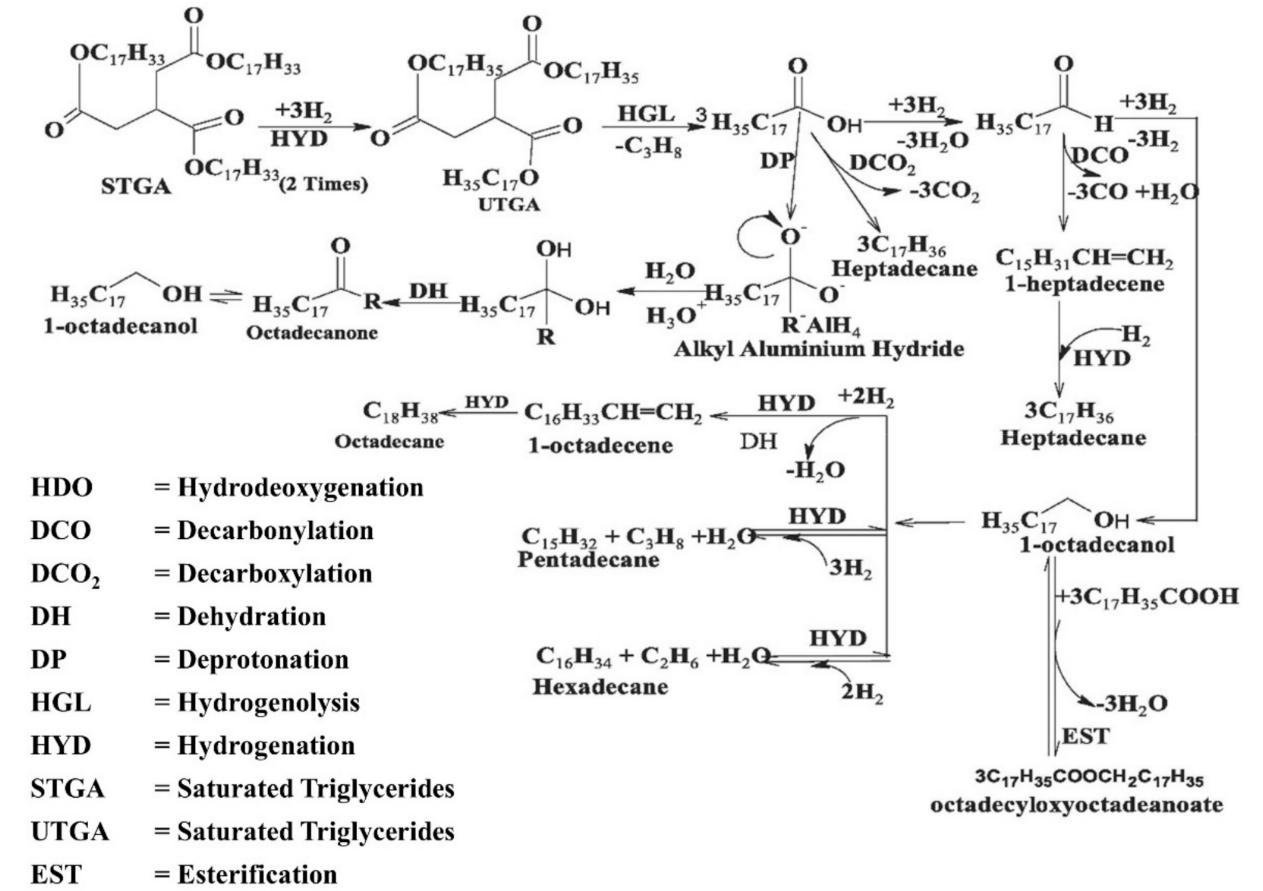
2. Renewable Diesel and Biodiesel Production
3. Activated Carbon as Catalyst Supports
| Type of Support | Elemental Composition | Surface Area | Pore Volume | Remarks | Reference | |
|---|---|---|---|---|---|---|
| AC | C: 90.03 % H: 0.557% N: 0.367% S: 0.069% O: 8.98% C/H: 161.6 |
Micropore: 775 m2/g External: 15 m2/g |
Micro: 0.23 cm3/g Total: 0.26 cm3/g |
Charcoals from Iwasaki kiln | [37] | |
| AC | C: 80.71 % H: 1.146% N: 1.094% S: 0.078% O: 16.97% C/H: 70.4 |
Micropore: 1202 m2/g External: 20 m2/g |
Micro: 0.39 cm3/g Total: 0.42 cm3/g |
Charcoals from tube furnace | [37] | |
| AC | - | BET: 1484.33 cm2/g | Total: 1.038 cm3/g | Acid sites: 3.96 mmol NH3/g catalysts | [45] | |
| AC | - | BET: 266.1 m2/g | Total: 0.17 cm3/g | Pre-treated with a nitric acid solution | [46] | |
| AC | C: 88.57 wt% O: 8.01 wt% P: 3.42 wt% |
BET: 350 m2/g | Total: 1.88 cm3/g | Total acidity (144 °C): 1055.3 µmol/g Total acidity (852 °C): 2064.7 µmol/g Total basicity (902 °C): 1086.6 µmol/g |
[47] | |
| AC | C: 79.1 w/w% H: 0.9 w/w% N: 0.9 w/w% O: 19.2 w/w% |
BET: 964 m2/g | Micro: 77.92% Meso: 22.08% Total: 0.57 cm3/g |
- | [48] | |
| Type of support | Type of catalyst | Composition of the active phase | Surface area | Pore volume | Remarks | Reference |
| AC | NiP | Ni: 5.14 wt% P: 2.23 wt% |
Micropore: 739 m2/g External: 15 m2/g |
Micro: 0.22 cm3/g Total: 0.25 cm3/g |
Charcoals from Iwasaki kiln | [37] |
| AC | NiP | Ni: 4.66 wt% P: 2.24 wt% |
Micropore: 851 m2/g External: 16 m2/g |
Micro: 0.26 cm3/g Total: 0.31 cm3/g |
Charcoals from tube furnace | [37] |
| AC | Ni2P | - | BET: 612 m2/g | - | Total acidity: 1.3 mmol/g | [49] |
| AC | Ni | O (on the surface): 9.4% | BET: 807.26 cm2/g | Total: 0.185 cm3/g | - | [45] |
| AC | Co-Fe | Co: 8.67 wt% Fe: 3.52 wt% |
Micropore: 459.91 m2/g | Micro: 0.22 cm3/g Total: 0.44 cm3/g |
- | [43] |
| AC | Mo2C | Mo(II): 52% Mo(IV): 8% Mo(VI): 40% |
Total: 417.02 m2/g | Total: 0.22 cm3/g | - | [50] |
| AC | Mo2C | Mo2C (II): 52.17% MoO2 (IV): 8.2% MoO3 (VI): 39.63% |
BET: 322.20 m2/g | Total: 0.202 cm3/g | - | [46] |
| AC | Co-Ag | C: 63.41 wt% O: 13.26 wt% P: 1.45 wt% Co: 9.57 wt% Ag: 12.31 wt% |
BET: 793 m2/g | Total: 1.67 cm3/g | Acidity: 8502.3 µmol/g Total basicity: 6220.2 µmol/g |
[47] |
| AC | CoP | - | BET: 822.9 m2/g | Micro: 68.79% Meso: 31.21% Total 0.43 cm3/g |
Acidity: 52.5 µmol/g | [48] |
4. Recyclability and Stability of Supported Catalysts
4.1. Recyclability
4.2. Stability
This entry is adapted from the peer-reviewed paper 10.3390/en15082835
References
- Khalit, W.N.A.W.; Askin-Mijan, N.; Marliza, T.S.; Gamal, M.S.; Shamsuddin, M.R.; Saiman, M.I.; Taufiq-Yap, Y.H. Catalytic deoxygenation of waste cooking oil utilizing nickel oxide catalysts over various supports to produce renewable diesel fuel. Biomass Bioenergy 2021, 154, 106248.
- Garraín, D.; Herrera, I.; Lechόn, Y.; Lago, C. Well-to-Tank environmental analysis of a renewable diesel fuel from vegetable oil through co-processing in a hydrotreatment unit. Biomass Bioenergy 2014, 63, 239–249.
- Patel, M.; Oyedun, A.O.; Kumar, A.; Gupta, R. A Techno-Economic Assessment of Renewable Diesel and Gasoline Production from Aspen Hardwood. Waste Biomass Valorizat. 2019, 10, 2745–2760.
- Aatola, H.; Larmi, M.; Sarjovaara, T.; Mikkonen, S. Hydrotreated Vegetable Oil (HVO) as a Renewable Diesel Fuel: Trade-off between NOₓ, Particulate Emission, and Fuel Consumption of a Heavy Duty Engine. SAE Int. J. Engines 2009, 1, 1251–1262.
- Kalnes, T.; Koers, K.P.; Marker, T.; Shonnard, D.R. Green Diesel and Biodiesel: A technoeconomic and Life Cycle Comparison. In Proceedings of the 1st Alternative Fuels Technology Conference, Prague, Czechoslovakia; 2008. Available online: https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/ep.10319 (accessed on 11 October 2021).
- Hill, S.; Shi, E.; Colletti, P.U.S.; Renewable Diesel Capacity Could Increase due to Announced and Developing Projects. Today in Energy. 2021. Available online: https://www.eia.gov/todayinenergy/detail.php?id=48916 (accessed on 13 October 2021).
- Nickel, R.; Kelly, S.; Plume, K. Plume Renewable Diesel Boom Highlights Challenges in Clean-Energy Transition. 2021. Available online: https://www.reuters.com/article/us-global-oil-biofuels-insight-idUSKBN2AV1BS (accessed on 11 October 2021).
- Niemantsverdriet, J.W. Spectroscopy in Catalysis: An Introduction; John Wiley & Sons: Weinheim, Germany, 2007; Printed in Federal Republic of Germany.
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938.
- Sakata, Y.; Tamaura, Y.; Imamura, H.; Watanabe, M. Preparation of a New Type of CaSiO3 with High Surface Area and Property as a Catalyst Support. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., De Vos, D.E., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 331–338.
- Chia, S.R.; Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.L. Renewable diesel as fossil fuel substitution in Malaysia: A review. Fuel 2022, 314, 123137.
- Gerhard, K.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook; AOCS Press: Urbana, IL, USA, 2010; Available online: https://www.sciencedirect.com/book/9781893997622/the-biodiesel-handbook?via=ihub=#book-description (accessed on 14 October 2021).
- Thangarasu, V.; Anand, R. Comparative evaluation of corrosion behavior of Aegle Marmelos Correa diesel, biodiesel, and their blends on aluminum and mild steel metals. In Advanced Biofuels; Azad, A.K., Rasul, M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Chapter 17; pp. 443–471.
- Ziolkowska, J.R. Biofuels technologies: An overview of feedstocks, processes, and technologies. In Biofuels for a More Sustainable Future; Ren, J., Scipioni, A., Manzardo, A., Liang, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 1; pp. 1–19.
- Venkatesan, M.; Vikram, C.J.; Naveenchandran, P. Performance and emission analysis of pongamia oil methyl ester with diesel blend. Middle East J. Sci. Res. 2012, 12, 1758–1765.
- Zahan, K.A.; Kano, M. Biodiesel Production from Palm Oil, Its By-Products and Mill Effluent: A Review. Energies 2018, 11, 2132.
- Zhang, Y.; Niu, S.; Han, K.; Li, Y.; Lu, C. Synthesis of the SrO–CaO–Al2O3 trimetallic oxide catalyst for transesterification to produce biodiesel. Renew. Energy 2021, 168, 981–990.
- Molina-Gutiérrez, M.; Alcaraz, L.; Lόpez, F.A.; Rodríguez-Sánchez, L.; Martínez, M.J.; Prieto, A. Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A. J. Fungi 2021, 7, 822.
- Ávila, S.N.S.; Collaço, A.C.A.; Greco-Duarte, J.; Aguieiras, E.C.G.; de Castro, A.M.; Gutarra, M.L.E.; Cavalcanti, E.D.C.; Freire, D.M.G. Development of a green integrated process for biodiesel esters production: Use of fermented macaúba cake as biocatalyst for macaúba acid oil transesterification. J. Am. Oil Chem. Soc. 2021, 98, 825–835.
- Kumar, R.; Pal, P. Lipase immobilized graphene oxide biocatalyst assisted enzymatic transesterification of Pongamia pinnata (Karanja) oil and downstream enrichment of biodiesel by solar-driven direct contact membrane distillation followed by ultrafiltration. Fuel Process. Technol. 2021, 211, 106577.
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31.
- European Technology and Innovation Platform. Hydrotreatment to HVO. 2021. Available online: https://www.etipbioenergy.eu/value-chains/conversion-technologies/conventional-technologies/hydrotreatment-to-hvo (accessed on 15 October 2021).
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Rashid, U.; Islam, A.; Taufiq-Yap, Y.H. A Review on Thermal Conversion of Plant Oil (Edible and Inedible) into Green Fuel Using Carbon-Based Nanocatalyst. Catalysts 2019, 9, 350.
- Papadopoulos, C.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. W promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Fuel Process. Technol. 2021, 217, 106820.
- Burimsitthigul, T.; Yoosuk, B.; Ngamcharussrivichai, C.; Prasassarakich, P. Hydrocarbon biofuel from hydrotreating of palm oil over unsupported Ni–Mo sulfide catalysts. Renew. Energy 2021, 163, 1648–1659.
- Nikolopoulos, I.; Kogkos, G.; Andriopoulou, C.; Kordouli, E.; Dracopoulos, V.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Cobalt–Alumina Coprecipitated Catalysts for Green Diesel Production. Ind. Eng. Chem. Res. 2021, 60, 18672–18683.
- Liu, P.; Chen, C.; Zhou, M.; Xu, J.; Xia, H.; Shang, S.; Jiang, J. Metal–organic framework-derived Ni-based catalyst for the hydrotreatment of triolein into green diesel. Sustain. Energy Fuels 2021, 5, 1809–1820.
- Lycourghiotis, S.; Kordouli, E.; Kordulis, C.; Bourikas, K. Transformation of residual fatty raw materials into third generation green diesel over a nickel catalyst supported on mineral palygorskite. Renew. Energy 2021, 180, 773–786.
- Fani, K.; Lycourghiotis, S.; Bourikas, K.; Kordouli, E. Biodiesel Upgrading to Renewable Diesel over Nickel Supported on Natural Mordenite Catalysts. Ind. Eng. Chem. Res. 2021, 60, 18695–18706.
- Ameen, M.; Azizan, M.T.; Ramli, A.; Yusup, S.; Alnarabiji, M.S. Catalytic hydrodeoxygenation of rubber seed oil over sonochemically synthesized Ni-Mo/γ-Al2O3 catalyst for green diesel production. Ultrason. Sonochem. 2019, 51, 90–102.
- Wisniowski, H.; Zhang, Y. Using Activated Carbon as a Precious Metal Catalyst Carrier. 2021. Available online: https://www.sigmaaldrich.com/MY/en/technical-documents/technical-article/materials-science-and-engineering/solid-state-synthesis/activated-carbon (accessed on 9 November 2021).
- Chandrasekhar, K. Chapter 3.5—Effective and Nonprecious Cathode Catalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 485–501.
- Nabihah-Fauzi, N.; Asikin-Mijan, N.; Ibrahim, M.L.; Hashim, H.; Yusup, S.; Taufiq-Yap, Y.H.; Mastuli, M.S. Sulfonated SnO 2 nanocatalysts via a self-propagating combustion method for esterification of palm fatty acid distillate. RSC Adv. 2020, 10, 29187–29201.
- Jin, W.; Pastor-Pérez, L.; Villora-Pico, J.J.; Pastor-Blas, M.M.; Sepúlveda-Escribano, A.; Gu, S.; Charisiou, N.D.; Papageridis, K.; Goula, M.A.; Reina, T.R. Catalytic Conversion of Palm Oil to Bio-Hydrogenated Diesel over Novel N-Doped Activated Carbon Supported Pt Nanoparticles. Energies 2020, 13, 132.
- Nie, R.; Yang, H.; Zhang, H.; Yu, X.; Lu, X.; Zhou, D.; Xia, Q. Mild-temperature hydrodeoxygenation of vanillin over porous nitrogen-doped carbon black supported nickel nanoparticles. Green Chem. 2017, 19, 3126–3134.
- Gamal, M.S.; Asikin-Mijan, N.; Khalit, W.N.A.W.; Arumugam, M.; Izham, S.M.; Taufiq-Yap, Y.H. Effective catalytic deoxygenation of palm fatty acid distillate for green diesel production under hydrogen-free atmosphere over bimetallic catalyst CoMo supported on activated carbon. Fuel Process. Technol. 2020, 208, 106519.
- Ruangudomsakul, M.; Osakoo, N.; Keawkumay, C.; Kongmanklang, C.; Butburee, T.; Kiatphuengporn, S.; Faungnawakij, K.; Chanlek, N.; Wittayakun, J.; Khemthong, P. Influential properties of activated carbon on dispersion of nickel phosphides and catalytic performance in hydrodeoxygenation of palm oil. Catal. Today 2021, 367, 153–164.
- Schröder, E.; Thomauske, K.; Weber, C.; Hornung, A.; Tumiatti, V. Experiments on the generation of activated carbon from biomass. J. Anal. Appl. Pyrolysis 2007, 79, 106–111.
- Schrder, E.; Thomauske, K.; Oechsler, B.; Herberger, S. Activated Carbon from Waste Biomass; InTech. Available online: https://www.intechopen.com/chapters/16653 (accessed on 9 November 2021).
- Edeh, I.; Overton, T.; Bowra, S. Catalytic hydrothermal deoxygenation of fatty acids over palladium on activated carbon catalyst (Pd/C) for renewable diesel production. Biofuels 2021, 12, 1075–1082.
- Tapia, J.; Acelas, N.Y.; Lόpez, D.; Moreno, A. NiMo-sulfide supported on activated carbon to produce renewable diesel. Univ. Sci. 2017, 22, 71–85.
- Safa Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Rashid, U.; Taufiq-Yap, Y.H. Solvent-free catalytic deoxygenation of palm fatty acid distillate over cobalt and manganese supported on activated carbon originating from waste coconut shell. J. Anal. Appl. Pyrolysis 2019, 144, 104690.
- Thangadurai, T.; Tye, C.T. Performance of Activated Carbon Supported Cobalt Oxides and Iron Oxide Catalysts in Catalytic Cracking of Waste Cooking Oil. Period. Polytech. Chem. Eng. 2021, 65, 350–360.
- Mayorga, M.; Cadavid, J.; Suarez, O.; Vargas, J.; Gonzalez, J.; Narvaez, P. Production of Renewable Diesel by Hydrotreating of Palm Oil with Noble Metallic Catalysts. Chem. Eng. Trans. 2019, 74, 7–12.
- Hongloi, N.; Prapainainar, P.; Seubsai, A.; Sudsakorn, K.; Prapainainar, C. Nickel catalyst with different supports for green diesel production. Energy 2019, 182, 306–320.
- Wang, F.; Jiang, J.; Wang, K.; Zhai, Q.; Sun, H.; Liu, P.; Feng, J.; Xia, H.; Ye, J.; Li, Z.; et al. Activated carbon supported molybdenum and tungsten carbides for hydrotreatment of fatty acids into green diesel. Fuel 2018, 228, 103–111.
- Safa-Gamal, M.; Asikin-Mijan, N.; Arumugam, M.; Khalit, W.N.A.W.; Nur Azreena, I.; Hafez, F.S.; Taufiq-Yap, Y.H. Catalytic deoxygenation by H2-free single-step conversion of free fatty acid feedstock over a Co-Ag carbon-based catalyst for green diesel production. J. Anal. Appl. Pyrolysis 2021, 160, 105334.
- Kaewtrakulchai, N.; Kaewmeesri, R.; Itthibenchapong, V.; Eiad-Ua, A.; Faungnawakij, K. Palm oil conversion to bio-jet and green diesel fuels over cobalt phosphide on porous carbons derived from palm male flowers. Catalysts 2020, 10, 694.
- Pham, L.K.H.; Tran, T.T.V.; Kongparakul, S.; Reubroycharoen, P.; Karnjanakom, S.; Guan, G.; Samart, C. Formation and activity of activated carbon supported Ni2P catalysts for atmospheric deoxygenation of waste cooking oil. Fuel Process. Technol. 2019, 185, 117–125.
- Wang, F.; Xu, J.; Jiang, J.; Liu, P.; Li, F.; Ye, J.; Zhou, M. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746.
- Malins, K. Synthesis of renewable hydrocarbons from vegetable oil feedstock by hydrotreatment over selective sulfur-free SiO2-Al2O3 supported monometallic Pd, Pt, Ru, Ni, Mo and bimetallic NiMo catalysts. Fuel 2021, 285, 119129.
- Alsultan, G.A.; Asikin-Mijan, N.; Lee, H.V.; Albazzaz, A.S.; Taufiq-Yap, Y.H. Deoxygenation of waste cooking to renewable diesel over walnut shell-derived nanorode activated carbon supported CaO-La2O3 catalyst. Energy Convers. Manag. 2017, 151, 311–323.
- Asikin-Mijan, N.; Rosman, N.A.; Abdulkareem-Alsultan, G.; Mastuli, M.S.; Lee, H.V.; Nabihah-Fauzi, N.; Lokman, I.M.; Alharthi, F.A.; Alghamdi, A.A.; Aisyahi, A.A.; et al. Production of renewable diesel from Jatropha curcas oil via pyrolytic-deoxygenation over various multi-wall carbon nanotube-based catalysts. Process Saf. Environ. Prot. 2020, 142, 336–349.
- Nur Azreena, I.; Lau, H.L.N.; Asikin-Mijan, N.; Hassan, M.A.; Izham, S.M.; Safa Gamal, M.; Nor Adira Wan Khalit, W.; Arumugam, M.; Kennedy, E.; Stockenhuber, M.; et al. Hydrodeoxygenation of fatty acid over La-modified HZSM5 for premium quality renewable diesel production. J. Anal. Appl. Pyrolysis 2022, 161, 105406.
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, S.; Polychronopoulou, K.; Goula, M.A. Effect of operating parameters on the selective catalytic deoxygenation of palm oil to produce renewable diesel over Ni supported on Al2O3, ZrO2 and SiO2 catalysts. Fuel Process. Technol. 2020, 209, 106547.
- Li, G.; Li, N.; Yang, J.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable diesel with the 2-methylfuran, butanal and acetone derived from lignocellulose. Bioresour. Technol. 2013, 134, 66–72.
- Pérez, W.; Marín, J.; del Río, J.; Peña, J.; Rios, L. Upgrading of palm oil renewable diesel through hydroisomerization and formulation of an optimal blend. Fuel 2017, 209, 442–448.
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, A.A.; AlKhoori, S.I.; Polychronopoulou, K.; Goula, M.A. Continuous selective deoxygenation of palm oil for renewable diesel production over Ni catalysts supported on Al2O3 and La2O3–Al2O3. RSC Adv. 2021, 11, 8569–8584.
