Carotenoids are synthesized de novo by organisms from all three major categories (domains) of life, i.e., eukaryotes (such as plants, algae, and some fungi), bacteria, and archaea [
1]. Animals, however, must consume carotenoids with their diet, except for the special case of some aphids that acquire carotenoid biosynthesis genes via transfer from fungi [
4]. Carotenoids are classified as either carotenes (pure hydrocarbons without oxygen, e.g.,
β-carotene) or xanthophylls (that contain oxygen, e.g., lutein and zeaxanthin) and absorb visible light of blue or blue-green wavelengths (resulting in carotenoids' yellow, orange, or red colors). The interaction of carotenoids, or their derivatives, with visible light gives rise to key functions in light absorption for both humans and plants. In human vision, the light-absorbing component of the protein rhodopsin is derived from carotenoids with provitamin A activity [
5]. In photosynthetic organisms, carotenoids have widespread functions that support photosynthesis, with a particularly prominent ecological role of green-light absorbance by fucoxanthin, siphonoxanthin, and peridinin in some algae [
6,
7,
8,
9]. Moreover, zeaxanthin and lutein in particular have vital functions in protection against damage by intense light in both plants (e.g., [
10]) and the human eye [
11].
2. Xanthophylls in High-Stress Contexts
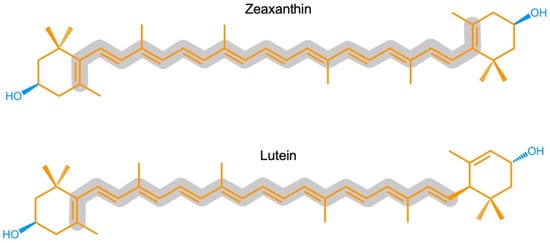
Zeaxanthin and lutein are structural isomers with zeaxanthin possessing a slightly longer system of conjugated double bonds (11) than lutein (10; Figure 1).
Figure 1. Chemical structures of zeaxanthin and lutein. Hydrophobic portions are shown in orange and hydrophilic portions in blue (containing oxygen). The grey outline highlights the longer continuous, conjugated system of carbon-carbon (C=C) double bonds in zeaxanthin compared to lutein.
2.1. Zeaxanthin and Lutein in the Human Eye/Retina
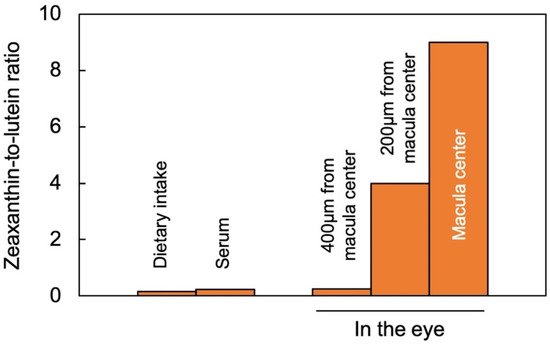
Lutein and zeaxanthin are differentially distributed across the human retina (
Figure 2). The yellow center of the eye (macula), where the brightest light is received, has the highest overall xanthophyll concentration and the highest ratio of zeaxanthin to lutein [
11]. The total xanthophyll concentration is about 1 mM in the macula and declines to less than 10 μΜ in the peripheral regions of the retina [
23]. In addition to zeaxanthin and lutein, meso-zeaxanthin (a zeaxanthin stereoisomer) is present in the macula and is apparently produced from dietary lutein but not from dietary zeaxanthin [
24].
A recent study using confocal resonance Raman spectroscopy, validated by biochemical characterization of carotenoid composition, described the variation in the zeaxanthin-to-lutein ratio over short distances using continuous scans of xanthophyll composition across donor retinas [
25]. The zeaxanthin-to-lutein ratios were 9:1 or greater in the center of the macula; 4:1 at a short distance (200 µm) from the center; and 1:4 just outside the macula (
Figure 2; [
25]). This preferential placement of zeaxanthin where the brightest light is received indicates a unique role of zeaxanthin in supporting the vision process in the presence of bright light. Still, this finding does not allow an assessment of which one(s) of the multiple possible roles of zeaxanthin is/are at work in this location. Original ideas (starting in 1861) about the function of the xanthophyll-rich macula initially centered on potential improvements in visual acuity and contrast sensitivity with reduced glare sensitivity and light scatter (see [
11]). However, the subsequent rise of age-related macular degeneration in the human population shifted the focus of attention to photoprotection (see review [
11]). Nevertheless, both principal roles are still discussed today, and multiple mechanisms are under consideration (see below).
Figure 2. Zeaxanthin-to-lutein ratios across a gradient from dietary intake to blood serum and different locations in the human eye, after data from [
26] for dietary intake and serum, and for the areas within and around the macula from [
25]. “Zeaxanthin” here represents the sum of zeaxanthin and meso-zeaxanthin (present in a 1;1 molar ratio in the macula).
2.2. Zeaxanthin and Lutein in Leaves
Leaves of plants growing in sunny locations under conditions favorable for growth rapidly form and remove zeaxanthin as the fraction of absorbed light not utilized in photochemistry rises and falls over the course of the day (e.g., [
10,
27]). Characterizations of the latter functional features was enabled by the development of portable instruments to measure chlorophyll fluorescence from leaves under field conditions and in the presence of bright light [
28,
29]. Zeaxanthin is formed in the presence of excess absorbed light from the di-epoxide violaxanthin via the mono-epoxide antheraxanthin in the xanthophyll cycle [
30,
31]. Violaxanthin levels exhibit complementary decreases and increases over the course of the day (e.g., [
10,
27]). However, the levels of other ubiquitous leaf carotenoids (lutein,
β-carotene, and neoxanthin) do not typically change over the course of the day in sun-exposed habitats [
10]. In slow-growing evergreens (that utilize only a low fraction of full sunlight for photochemistry), the ratio of zeaxanthin to lutein can approach unity in full sun at midday, but this ratio is much lower in most plant systems most of the time. Ample zeaxanthin for human nutrition is thus hard to come by when relying on rapidly growing leafy greens, harvested and then stored before consumption. For human nutrition, crops that combine rapid growth
and high zeaxanthin levels would be desirable.
We recently reported on the unusual ability of aquatic floating plants (Lemnaceae, or duckweeds) to simultaneously grow very rapidly
and accumulate exceptionally high levels of zeaxanthin [
32,
33,
34].
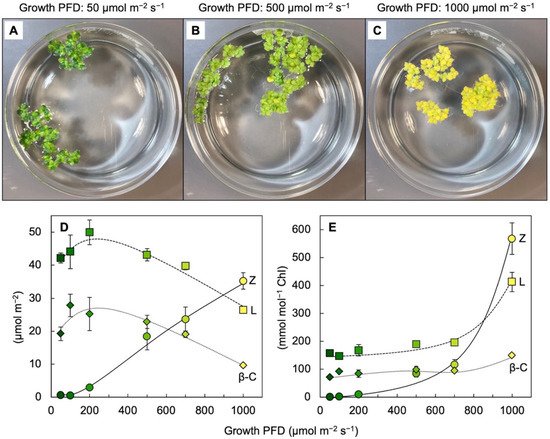
Figure 3 shows the visual appearance and carotenoid levels for
Lemna grown under a wide range of photon flux densities (PFDs) in which plant growth remained high, with plant area doubling every other day. Additionally, zeaxanthin levels continued to rise when absorbed light became increasingly excessive, whereas the levels of chlorophyll, lutein, and
β-carotene declined (
Figure 3D). The plants grown under the highest light intensity were bright yellow (
Figure 3C), still grew very rapidly, and exhibited zeaxanthin-to-lutein ratios as high as ~1.3 (up from ~0.5 under the next lowest growth PFD). Due to the fact that the levels of chlorophyll
a +
b declined more sharply than those of any of the carotenoids under the highest-growth PFD, carotenoid levels increased relative to chlorophyll, and none more sharply than zeaxanthin (
Figure 3E). A considerable portion of this zeaxanthin is presumably dissolved in the phospholipid portion of chloroplast membranes. A role for zeaxanthin, but not lutein, as a membrane-based antioxidant and/or membrane stabilizer was proposed for plants [
35,
36]. The sharp increase in the zeaxanthin-to-lutein ratio in duckweed at the highest PFD (
Figure 3D) is reminiscent of the dynamics across the human eye described above.
Figure 3. (
A–
C) Images of crystallizing dishes with
Lemna gibba fronds grown under 3 different PFDs. Relationship between growth PFD and the levels of zeaxanthin (Z), lutein (L), and
β-carotene (
β-C) on a frond area basis (
D) or a chlorophyll basis (
E). Colors of the symbols from dark green to yellow correspond to the different visual appearances of fronds grown under the different growth PFDs. Data re-graphed from [
33].
The unusual ability of duckweeds to
combine pronounced zeaxanthin accumulation with fast growth may be associated with (i) exposure of the entire leaf cross-section to excess light due to minimal self-shading (in the absence of fixed multi-layer plant canopies or thick leaves [
32,
33,
34]) and (ii) the loss of controls that act on growth in land plants [
37]. This unusual combination may also apply to other fast-growing edible aquatic plants [
32], which could be of considerable interest to human nutrition and illustrates the importance of species choice. Although whole-food sources with superior zeaxanthin content are of interest to human nutrition, it should be noted that supplements (e.g., the AREDS2 formulation with 10 mg lutein and 2 mg zeaxanthin) can also provide benefits, such as delayed progression to advanced macular degeneration [
38].
In addition to offering photoprotection in high-light environments, zeaxanthin also plays a role in the heat tolerance of plants. A greater heat tolerance was demonstrated for
Arabidopsis thaliana lines engineered to overexpress
β-hydroxylase (the enzyme catalyzing conversion of
β-carotene to zeaxanthin). This engineered line exhibited elevated zeaxanthin levels but no enhancement of non-photochemical dissipation excess light, which is consistent with different roles of additional zeaxanthin in the phospholipid portion of the photosynthetic membrane [
39].
2.3. Zeaxanthin and Related Xanthophylls in Extremophiles
Among the over 1100 naturally occurring carotenoids described, only seven are synthesized de novo by organisms from all three domains of life [
1]. The few known carotenoids synthesized by representatives of eukaryotes, bacteria, and archaea include zeaxanthin and its biosynthetic precursors (for a detailed review of carotenoid biosynthetic pathways among the taxa of life, see [
40]). Zeaxanthin is found not only in light-absorbing/photosynthetic bacteria but also in non-photosynthetic bacteria and archaea. Although the functions of zeaxanthin and related xanthophylls (
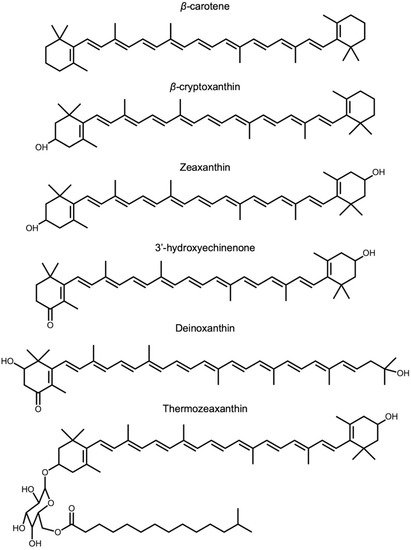
Figure 4) in these organisms are yet to be elucidated, the environments in which they occur expose these organisms to high levels of stress (visible or ionizing radiation, heat, or salinity). Among eukaryotes, fungi typically do not produce lutein or zeaxanthin but can produce a variety of other carotenoids [
41,
42] as well as many other pigments (e.g., melanins, flavins, phenazines, quinones, monascins, violacein, and indigo; [
43]).
Figure 4. Chemical structures of β-carotene, β-carotene-derived structures, and structures with similar features that are mentioned in the text, i.e., β-carotene β-cryptoxanthin, zeaxanthin, 3′-hydroxyechinenone, deinoxanthin, and thermozeaxanthin.
Zeaxanthin and related xanthophylls accumulate in bacteria and archaea that occur in the most extreme environments. Prokaryotic organisms do not synthesize lutein [
42] but do produce zeaxanthin. Zeaxanthin-producing microorganisms include photosynthetic cyanobacteria, such as
Synechococcus (see [
44]) that accumulates zeaxanthin in the high-light environment of surface ocean water, but less so in sub-surface layers where light levels are lower [
45]. Cyanobacteria use other xanthophylls, such as 3′-hydroxyechinenone (
Figure 4), to protect their phycobilisome chromophores [
46] that harvest light in blue- and red-light-depleted zones deeper in the water column.
Zeaxanthin is also accumulated by non-photosynthetic bacteria, some of which are named after this feature (
Mesoflavibacter zeaxanthinifaciens,
Zeaxanthinibacter enoshimensis,
Paracoccus zeaxanthinifaciens; [
47,
48]). A notable case of an extremophile is the bacterium
Deinococcus radiodurans, isolated from a radioactive site in Japan and described as a “gold medalist” for tolerance of ionizing radiation [
49].
Deinococcus radiodurans accumulate deinoxanthin and zeaxanthin [
50,
51,
52,
53]. Ionizing radiation produces vast amounts of ROS (e.g., hydroxyl radical and superoxide) via water hydrolysis [
54]. A zeaxanthin glycoside ester, thermozeaxanthin, is accumulated in non-photosynthetic microorganisms, including members of the bacterial genera
Halococcus,
Halobacterium, and
Thermus [
55,
56] as well as the Archean
Haloarcula japonica [
57], which all exhibit robust resistance to salinity and/or extreme heat. These findings further support a role of xanthophylls with features such as those shown in
Figure 4 in supporting life in extreme environments.
3. Association with Proteins and/or Phospholipid Bilayers
3.1. Association of Lutein and Zeaxanthin with Proteins—Selected Examples across Taxa
Carotenoids bind to a wide variety of proteins, including some with chromophores that intercept light and many that do not interact with light. In photosynthetic organisms, most light-harvesting proteins bind carotenoids in addition to their primary light-collecting chromophores (see chapters on carotenoid association with light-collecting complexes in various photosynthetic organisms in [
58]). Ongoing work on photosynthetic organisms continues to expand the list of carotenoid-binding proteins hat either bind light-harvesting pigments or interact with light-harvesting complexes. Many of these proteins bind lutein and/or zeaxanthin (see, e.g., [
59]) or other xanthophylls (see, e.g., [
60] for the orange carotenoid-binding protein of cyanobacteria).
Similarly, proteins involved in human vision (such as retinoid transporter proteins that have indirect roles in the vision process) bind zeaxanthin and lutein [
38]. Moreover, selective uptake of zeaxanthin and lutein into the macula of the human retina is mediated by two different proteins [
61] that bind either zeaxanthin and meso-zeaxanthin (glutathione S-transferase protein [
62]) or lutein (steroidogenic acute regulatory domain protein [
63]). Carotenoid-binding proteins not associated with light-collecting processes include proteins that transport carotenoids through the bloodstream in humans, such as high-density lipoprotein (for an in-depth review of human proteins that bind carotenoids, especially lutein and zeaxanthin, see [
38]).
3.2. Lutein and Zeaxanthin Localization within the Phospholipid Bilayer of Biological Membranes—Selected Examples across Taxa
Carotenoids may have first emerged in archaea as molecules that reinforced biological membranes as “molecular rivets” with just the right length and structure to span the phospholipid bilayer [
18,
64]. Carotenoids are also localized in membranes in many other organisms. Xanthophylls, in particular, can incorporate directly into phospholipid bilayers in a membrane-spanning orientation with no apparent association with proteins and do so in some microorganisms (see above), plants [
34,
36], and humans. The high levels of carotenoids in the human brain (71% of which consists of the xanthophylls lutein, zeaxanthin, and cryptoxanthin [
65]) are likely localized largely in the phospholipid bilayer of membranes. Although it seems clear that lutein is a component of the lipid bilayer portion of animal membranes, localization in the lipid bilayer portion of plant membranes has thus far been discussed mainly for zeaxanthin [
35,
36]. Future research should further address if lutein also plays a role in plant membranes, and if not, why.
In vitro studies demonstrated that lutein and zeaxanthin have different orientations in phospholipid bilayers. Whereas zeaxanthin was exclusively orientated in a perpendicular, membrane-spanning orientation, some of the lutein was oriented in a horizontal position parallel to the phospholipid head groups [
66,
67]. On the other hand, lutein may have a higher propensity to form tightly stacked aggregates that exhibit a blue shift in xanthophyll absorbance, which may affect the absorption of blue light in the retina [
68]. Moreover, biological membranes are clearly heterogeneous along their axes, with some microdomains containing more polyunsaturated fatty acids (PUFAs) and others more saturated fatty acids and cholesterol; xanthophylls are concentrated in the areas enriched in PUFAs [
69,
70]. More work is needed to ascertain similarities and differences in zeaxanthin and lutein localization and/or orientation in microdomains and in membranes as well as the functional significance of such differences.