1. Desolvation
Desolvation is the most frequently used technique to formulate protein-based nanoparticles, due to simplicity of operations, mild conditions, and the ability to obtain small particle sizes [
39].
In brief, the dropwise addition of a desolvation agent to an aqueous solution of protein reduces the availability of water molecules and causes dehydration of polymer chains. When the hydration becomes too low, chains aggregate, and the protein precipitates in the form of nanoparticles. Sometimes, nanoparticles are not stable; therefore, crosslinking agents must be added [
15,
40].
Li et al. prepared keratin nanoparticles loaded with doxorubicin (DOX) via the desolvation technique, using ethanol as a desolvation agent, followed by the electrostatic adsorption of the drug [
41]. The keratin used in this work was extracted from human hair, and then nanoparticles were prepared. In brief, they added ethanol to the aqueous protein solution in order to decrease the solubility of keratin until the formation of nanoparticles. Then, they used glutaraldehyde to crosslink the nanoparticles to make them stable in solution. Finally, DOX-loaded keratin nanoparticles (KDNPs) were formed by suspending keratin nanoparticles (KNPs) in DOX solution; thus, the drug loading content (LC) and drug encapsulation efficiency were evaluated. Keratin nanoparticles were characterised in terms of size by DLS and SEM, showing a diameter of around 214.8 nm and 150 nm, respectively. On the other hand, KDNPs were proven to have similar diameters: 220.8 nm by DLS, and around 150 nm by SEM.
Since the purpose of their work was to prepare a drug delivery system based on protein nanoparticles, the authors also studied the release of DOX under physiological conditions (PBS pH 7.4) and in acidic environments, in order to simulate the pH of the tumour area. At first, the pharmaceutical release seemed to be quite slow at both pH levels; subsequently, the release took two different paths: at pH 7.4 only 35% of DOX was released, while at pH 5.3 around 50% was released, due to the protonation of both DOX and keratin. The authors also studied the drug release profile depending on glutathione (GSH) concentration, showing that more than 60% of DOX was released at the GSH level of the tumour environment, while in the absence of GSH the release was only about 18%. Finally, the MTT assay led Li et al. to demonstrate the non-toxicity of KNPs and the high antitumour efficiency of keratin nanoparticles charged with doxorubicin against lung cancer cells.
Photodynamic therapy (PDT) is a non-surgical clinical treatment that can be used for infections and malignant cancers [
42,
43]. This technique exploits the activity of photosensitizers (PSs), which can induce ROS formation upon irradiation with appropriate wavelengths of light [
44]. Reactive oxygen species cause cells’ proliferation to decline, and sometimes cause the death of cells [
45,
46]. The penetration of the light in the tissue was studied by Ash et al. [
47], and it was reported that red light penetrates at 4–5 mm. On the other hand, PSs have several side effects, i.e., lack of specificity and high tendency to aggregate in aqueous media [
48]; thus, in recent years, researchers have focused their attention on the development of loaded nanoparticles, which can act as delivery systems, transporting the drug to the desired site and promoting its absorption [
49]. Several studies have reported that the penetration of the light is also enough to activate nanoparticles—i.e., keratin-based ones—loaded with different photosensitizers into the tumour within the bone tissue [
50,
51].
In this context, for the first time, Aluigi et al. described the preparation via desolvation of keratin nanoparticles conjugated with chlorin e6 (Ce6)—a second-generation photosensitizer [
14]. In this regard, keratin was extracted from Merino wool as previously described [
52], and Ce6 was covalently bound to the keratin amino groups via EDC/NHS coupling in DMSO as a solvent. The protein conjugated with Ce6 was purified by dialysis against NaHCO
3 buffer for 2 days, and finally freeze-dried to obtain a powder. In order to prepare Ker–Ce6 nanoparticles (KNPs@Ce6), ethanol was used as a desolvation agent, because it is able to decrease the protein solubility. Firstly, the powder was solubilised in NaHCO
3 buffer, and then ethanol was added under constant stirring at room temperature to precipitate nanoparticles. Therefore, glutaraldehyde (8%) was added to stabilise KNPs@Ce6, and the solution was purified by three cycles of centrifugation in water. This procedure allowed stable nanoparticles with hydrodynamic diameters around 147 nm and a Ce6 loading ratio of about 45% to be obtained, which were evaluated through UV–Vis measurements on nanoparticle suspensions. Further characterisations showed that KNPs@Ce6 displayed an excellent ability to cross the tumour cell membranes in both osteosarcoma and glioblastoma, and to produce a large amount of reactive oxygen species (ROS) upon light irradiation at the tumour site [
53].
Similarly, Kundu et al. reported the preparation of silk-fibroin-based nanoparticles via the desolvation method [
54]. In brief, silk fibroin (SF) aqueous solution was obtained by extraction from
Bombyx mori cocoons using a standard procedure [
55]. SF solution was added dropwise to DMSO solution under constant low magnetic stirring; the formation of nanoparticles was confirmed by the precipitate produced at the bottom. Then, nanoparticles were separated and purified by centrifugation with deionised water. Hence, they were redispersed in deionised water by sonication, and finally filtered using a 0.45 µm syringe filter to remove dust particles or contaminants. Finally, fluorescein isothiocyanate was linked to SF nanoparticles. The nanoparticles obtained had negative charges on the surface, as demonstrated by the reported value of zeta potential (−24.41 mV), which prevented further agglomeration of the particles; therefore, they were stable in deionised water and in cell culture media containing serum over a long period of time. FTIR spectra demonstrated that, during the formation of nanoparticles, the conformation of silk went from I to II, explaining the nanoparticles’ insolubility. A light-scattering particle analyser demonstrated that the nanoparticles’ hydrodynamic diameter was around 177 nm, and TEM analysis showed that the particles were spherical granules without signs of adhesion. Cytotoxicity and cell cycle assays on murine fibroblast cells were also carried out, and demonstrated that silk fibroin nanoparticles were moderately non-toxic to the cells. Overall, this study shows a smart methodology to fabricate silk fibroin nanoparticles, which can be used for drug delivery.
Additionally, Zhang et al. reported the preparation of silk fibroin nanoparticles starting from an aqueous solution of regenerated SF, which was mixed with water-miscible protonic organic solvents (e.g., ethanol and methanol) or with aprotic organic solvents (e.g., acetone) [
56]. The formation of nanoparticles depends on the configuration transition from random coils and α-helices to β-sheets. Briefly, the regenerated SF solution, obtained by extraction from
Bombyx mori cocoons, was quickly added to at least 70% (
v/
v) of the final mixture volume of water-miscible organic solvent—specifically, acetone. This process caused an increase in the number of β-sheet structures; therefore, the protein started to precipitate, and nanoparticles were formed. Then, the particles were purified by centrifugation, redispersed in deionised water, and finally lyophilised. TEM and SEM analysis showed that silk fibroin nanoparticles were globular granules with some microparticles aggregating together, and they were in the range of 35–125 nm in diameter. Finally, the authors demonstrated that the presence of SF nanoparticles did not affect the growth and propagation of bacteria, whether Gram-positive or Gram-negative.
2. Electrospraying
Electrospraying is a suitable technique to prepare micro- and nanoparticles using various materials, such as natural biopolymers. This process is based on the use of an electric field, which is applied to a drop, generating an electric charge, called Coulomb force. When this force becomes greater than the cohesive force, a diminution in the surface tension occurs, and nanoparticles are obtained [
57,
58]. Over time, variations on the conventional electrospraying technique have been developed.
Moreover, electrospraying is widely used due to its facility, simple control of parameters, and the possibility to obtain products in one step. In the literature, numerous studies have reported the synthesis of protein nanoparticles via electrospraying methods in order to develop alternative drug delivery systems, since this technique allows drugs to be encapsulated into protein nanoparticles [
65,
66,
67].
In this regard, in 2014, Ebrahimgol et al. documented the preparation of keratin nanoparticles by electrospraying, starting from Merino wool [
68]. In particular, keratin was extracted from wool using reduction hydrolysis [
69], which enables a powder of protein to be obtained as a result of the freeze-drying process. Keratin was then solubilised in formic acid at 70 °C for 24 h under magnetic stirring, using a concentration about 0.3%
w/v. The solution was placed into a syringe with a needle, and then connected to a positive electrode, while the aluminium foil collector was connected to the negative electrode and, finally, high voltage was applied. The authors also studied several concentrations of keratin solution, concluding that when the concentration was higher than 0.5%, the solution could not be electrosprayed. SEM images showed that the nanoparticles were spherical and their diameter was around 36–72 nm, depending on the electrospraying conditions. Then, Ebrahimgol et al. evaluated the effect of nozzle–collector distance for two feed rate levels (0.02 mL/h and 0.04 mL/h). Specifically, it was discovered that the dimensions of the particles decreased as the nozzle–collector distance increased and as the feed rate decreased, while they were not influenced by the applied voltage. Collectively, this paper showed a new smart methodology to prepare keratin nanoparticles in a controlled manner.
Guo et al., in 2018 [
70], used an electrospraying technique to modify polymeric nanofibers’ surface with keratin nanoparticles (KNPs), in order to improve the hydrophilicity and biocompatibility of polyvinyl alcohol (PVA) nanofibers [
71]. In the literature, some studies have reported the preparation of keratin–PVA blended nanofibers, but the use of protein caused a lot of problems due to its low viscosity and poor spinning properties [
72,
73,
74]; therefore, the purpose of this work was to offer a strategy to overcome these drawbacks. Briefly, oxidative keratin (keratose, KOS) was extracted from human hair via an oxidation method [
72], and PVA nanofibers were electrospun using a 10% (
w/v) solution in ethanol. Then, the KNPs were directly sprayed onto the PVA nanofibers by electrospray deposition, using different amounts of keratose (1.0, 1.5, 2.0, and 2.5%). The process was performed on aluminium foil paper, and the nanofibers were dried at 50 °C for 24 h. SEM images demonstrated that the KOS nanoparticles’ diameters were approximately 250–350 nm, and that they adhered to the PVA nanofibers. Additionally, KNP–PVA nanofibers showed improved performance in neural cell morphology, adhesion, and proliferation, depending on the presence of KNPs, compared to pure PVA nanofibers. Therefore, Guo et al. demonstrated the possibility to functionalise polymeric nanofibers with KNPs by using electrospray deposition in order to improve the biocompatibility and mechanical properties for potential neural tissue applications.
In 2014, Qu et al. [
75] demonstrated the preparation of silk fibroin nanoparticles (SFN) by electrospraying to produce controlled-release carriers of cisplatin (CDDP)—a chemotherapeutic agent—in order to reduce its cytotoxicity and adverse effects on healthy tissue. Silk fibroin (SF) was extracted from raw silk fibres as previously described by Wang et al. [
76], obtaining an aqueous protein solution. Then, glycerin was added to the SF solution (30% vs. protein). SFNs were prepared by electrospraying, using different electrostatic voltages (between 13 and 16 kV), and the droplets were continuously collected and frozen in a liquid nitrogen bath. SFNs were freeze-dried, redispersed in deionised water, and centrifuged in order to remove impurities. CDDP was then incorporated into SF nanoparticles via a ligand-exchange reaction of Pt from the chloride to the carboxyl group in the SF structure.
Briefly, an amount of cisplatin powder (about 20% w/w) was added to an aqueous nanoparticle solution, and then the system was stirred and, finally, dialysed. The encapsulation efficiency and the drug loading content were about 87.4% and 11.4%, respectively. SEM images allowed the researchers to demonstrate that the SFNs obtained were all spherical and well dispersed, while their diameters varied with the voltage. In particular, nanoparticles prepared under 14 kV and 15 kV showed smaller diameters than those obtained under 13 and 16 kV, probably due to the instability of the jet flow. Then, in vitro release of CDDP-loaded nanoparticles demonstrated that the cisplatin was released slowly, allowing a decrease in unwanted effects on healthy tissues. In vitro cellular cytotoxicity tests on murine fibroblast cells confirmed that CDDP–SFN had no apparent toxic effect on normal cells, while free CDDP displayed strong toxicity to them. Finally, these nanoparticles demonstrated a noteworthy inhibitory effect on lung cancer cells, which they could penetrate via adsorption endocytosis.
Similarly, Cao et al. [
77], in 2017, proposed the coaxial electrospraying technique to produce a novel potential drug delivery system for doxorubicin (DOX), based on core–shell PVA–silk fibroin nanoparticles. This method used two distinct capillaries: the PVA ethanol solution, containing DOX, was injected into the inner channel, while the SF solution, extracted from
Bombyx mori cocoons and then dissolved in HFIP, was injected into the outer channel. The authors studied the effect of PVA content on nanoparticle dimensions, concluding that the size diameters were controlled by polymer concentrations in solution. In particular, particles became larger as the concentration of the polymer increased. Moreover, DOX–PVA/SF NPs were negatively charged on the surface; hence, they were stable in solution. TEM images showed the core–shell structure of particles with an inner core of DOX–PVA covered by a layer of SF. Cao et al. also studied the DOX release profile, showing that the inner PVA core was dissolved into the aqueous medium, initially facilitating the drug release. After the initial burst release, it seemed that several DOX molecules remained entrapped by SF; therefore, the release was facilitated by the use of ultrasound stimuli. Finally, the authors evaluated the cytotoxicity of the nanoparticles, finding that PVA/SF NPs were safe for human breast cancer cells, while DOX–PVA/SF NPs had high cytotoxicity to tumour cells when doxorubicin was released.
3. Self-Assembly and/or Aggregation
Self-assembly, also sometimes called aggregation when mediated by drugs, is a suitable technique used in the field of nanotechnology, because it allows stable nanometre products to be obtained under mild conditions, and without involving organic solvents or high temperatures. This process occurs when a disordered system spontaneously forms a well-organised structure as a result of specific local interactions between species [
78,
79,
80]. The main forces involved in this process are van der Waals, hydrophobic, electrostatic, and hydrogen bonding forces, which are generally weak, but when combined together allow stable self-assembled structures to be obtained [
81]. The self-assembly technique can be classified as follows [
78]:
- -
-
Static: occurs in the absence of external factors, and is determined by the minimisation of energy;
- -
-
Dynamic: the process is influenced by external factors.
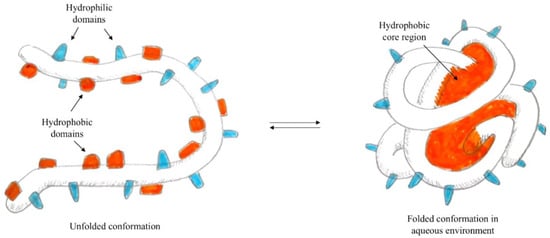
In particular, when the self-assembly technique is used for the preparation of polymeric nanoparticles—i.e., proteins [
82]—the presence of both hydrophilic and hydrophobic domains is exploited. In fact, in water, the proteins form micelles, where hydrophobic domains face the core while hydrophilic domains orient on the surface, as represented in
Figure 1. Using the formation of interactions between hydrophobic residues of the protein and lipophilic molecules, it is possible to load the hydrophobic core of the nanoparticle with active lipophilic principles, which in their free form are insoluble in an aqueous environment.
Figure 1. Schematisation of protein self-assembly.
In this regard, Liu et al. [
83] reported the preparation of human hair keratin (HHK) nanoparticles loaded with mupirocin (MPC)—a novel antibacterial agent with a mode of action different from other antibiotic agents—allowing them to obtain a nano drug delivery system. Briefly, keratin was extracted from human hair by slightly modifying the procedures reported in [
84], and a protein aqueous solution (10 wt%) was obtained. Meanwhile, different amounts of MPC were dissolved in PBS buffer solution. Then, the nanoparticles were formed by mixing HHK solution and MCP solution under stirring at pH 6.5. In this work, the concentrations of both keratin and MCP solutions were studied, and the researchers noted that the formation of nanoparticles did not occur when the concentration of HHK or MCP was higher than 0.5%. The dynamic light-scattering particle size analyser showed that nanoparticle diameters were about 75 nm, while SEM images confirmed that the keratin nanoparticles loaded with mupirocin had an irregular shape. In conclusion, Liu et al. demonstrated the preparation of keratin nanoparticles using self-assembly and electrostatic interaction, in order to suggest a novel drug delivery system based on a natural biopolymer.
Recently, in 2021, Du et al. [
85] investigated the preparation of keratin–tannic acid complex nanoparticles, to produce a pH/GSH dual-responsive drug carrier for doxorubicin. In particular, tannic acid (TA) is usually employed as a non-toxic crosslinker for proteins [
86,
87], and in this work it formed complex nanoparticles together with keratin via a self-assembly process with non-covalent interactions, including hydrogen bonding and hydrophobic interaction [
88]. Firstly, keratin was extracted from human hair using the reduction method described in [
89]. Then, tannic acid powder was added to the aqueous keratin solution, and the system was stirred for 12 h at room temperature. The mixture was then dialysed and lyophilised in order to obtain keratin–TA nanoparticles (KNPs). Finally, the drug-loaded keratin nanoparticles (DKNPs) were prepared by exploiting the hydrophobic interactions and hydrogen bonds between KNPs and doxorubicin. TEM images showed that the nanoparticles were round/oval and well dispersed. Additionally, further characterisation showed that the diameters were about 240 nm and the zeta potential was −0.21 mV. Moreover, the authors studied the drug release profile, demonstrating that DKNPs released more doxorubicin at pH 5.0 than at pH 7.4, due to the protonation of keratin, and also that the release of the drug was facilitated by high levels of GSH, which characterise the tumour area. The MTT assay, conducted on murine fibroblast cells, demonstrated that the toxicity of the free drug was higher than the cytotoxicity of DKNPs. Furthermore, the in vitro cytotoxicity test on lung carcinoma cells confirmed the antitumour activity of DKNPs, although their inhibition was weaker than that of free DOX, due to the different mechanism of interaction with the cells. Specifically, the free drug penetrated into the nucleus via diffusion, while DKNPs were internalised by endocytosis, and then released DOX.
For the first time, in 2018, Foglietta et al. [
90,
91] reported the application of keratin nanoparticles loaded with paclitaxel (PTX) as a novel drug delivery system (KER–NPs–PTX) to overcome the issues related to the use of taxanes, i.e., toxicity and poor water solubility [
92]. Briefly, keratin powder, obtained from wool as previously reported [
14], was dissolved in PBS solution, and then different amounts of PTX in ethanol were added under vigorous stirring. Characterisations of KER–NPs–PTX showed that the nanoparticles’ dimensions increased with PXT loading, ranging from 5 to 23% (wt). Additionally, TEM analysis showed that the nanoparticles were spherical, with a smooth surface morphology.
Stability studies in PBS and FBS/H2O indicated the high stability of nanoparticles, while cytotoxicity tests on human breast cancer cells demonstrated that KNPs were safe for cell lines. Finally, the authors studied KER–NPs–PTX’s activity against some different breast cancer models, showing the ability to inhibit tumour cell viability and to induce apoptosis. In summary, Foglietta et al. demonstrated the capacity of keratin to incorporate lipophilic drugs with high loading ratios, and the possibility of using KER–NPs as a novel drug delivery system.
Following the works reported above, Busi et al. [
93] studied the preparation via drug-mediated aggregation of keratin nanoparticles loaded with the R enantiomer of 9-hydroxystearic acid (9R), which exerted an in vitro growth-inhibitory effect on human colon carcinoma cells. In brief, the authors synthesised 9R as described in [
94], and then they added an ethanol solution of 9R to the aqueous solution of keratin under vigorous stirring, to obtain 9R-loaded keratin nanoparticles (9R@ker NPs). The 9R@ker NPs were characterised, demonstrating them to have an average diameter of around 160 nm and to be negatively charged in water, due to the SO
3− and COO
− groups of keratin. Moreover, the effect on human colon carcinoma cell proliferation was studied, concluding that 9R@ker NPs caused a decrease in cell proliferation comparable to the activity of the free drug.
This entry is adapted from the peer-reviewed paper 10.3390/nano12091406