Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Fucoxanthin, belonging to the xanthophyll class of carotenoids, is a natural antioxidant pigment of marine algae, including brown macroalgae and diatoms. It represents 10% of the total carotenoids in nature. The plethora of scientific evidence supports the potential benefits of nutraceutical and pharmaceutical uses of fucoxanthin for boosting human health and disease management.
- fucoxanthin
- seaweed
- bioactivities
- nutraceutical
- pharmaceutical
1. Structural Characteristics of Fucoxanthin
Fucoxanthin is a naturally occurring marine carotenoid, also known as marine xanthophyll, and is abundantly found as a pigment in the chloroplasts of brown algae. The structure of fucoxanthin is closely related to peridinin, neoxanthin, and dinoxanthin. Similar to other carotenoids, fucoxanthin obtains unique characteristic features of an unusual allenic bond, a 5,6-monoepoxide, nine conjugated double bonds, and oxygenic functional groups [1]. Moreover, the oxygenic functional groups consisting of epoxy, hydroxyl, carboxyl, and carbonyl moieties exceptionally express the superiority of fucoxanthin over other carotenoids (Figure 1). Moreover, the chemical formula of fucoxanthin is C42H58O6, which corresponds to a molecular weight of 658.9 g/mol. Fucoxanthin is readily vulnerable to any of the following factors, including light, heat, oxygen, enzymes, unsaturated lipids, and prooxidant molecules [1]. Depending on the treatment conditions, carrier molecules and carotenoid types may eventually cause cis-isomers formation by isomerization. Fucoxanthin extracted and purified from microalgae yielded three distinct peaks in which there are one trans-form and two cis-isomers in the chromatogram [1]. The trans-form of fucoxanthin is closely related to fucoxanthin’s pharmacological activities, as evidenced by previous studies. In a study conducted by Kawee-ai et al. [2], the antioxidant activities were decreased with the increase of cis-isomers formation. The spectrophotometric analysis of fucoxanthin-rich canola oil was subjected to different temperature exposures (between 25 and 100 °C) without light and air, resulting in the degradation of all-trans isomers of fucoxanthin, rather increasing 13-cis and 13′-cis isomers. This process followed simple first-order kinetics. Moreover, a similar pattern of degradation of fucoxanthin was observed when exposed to light and air. All studies shed light on the background knowledge of the structural properties of fucoxanthin, with the intent of taking preventive measures against chemical degradation when used for pharmaceutical purposes.
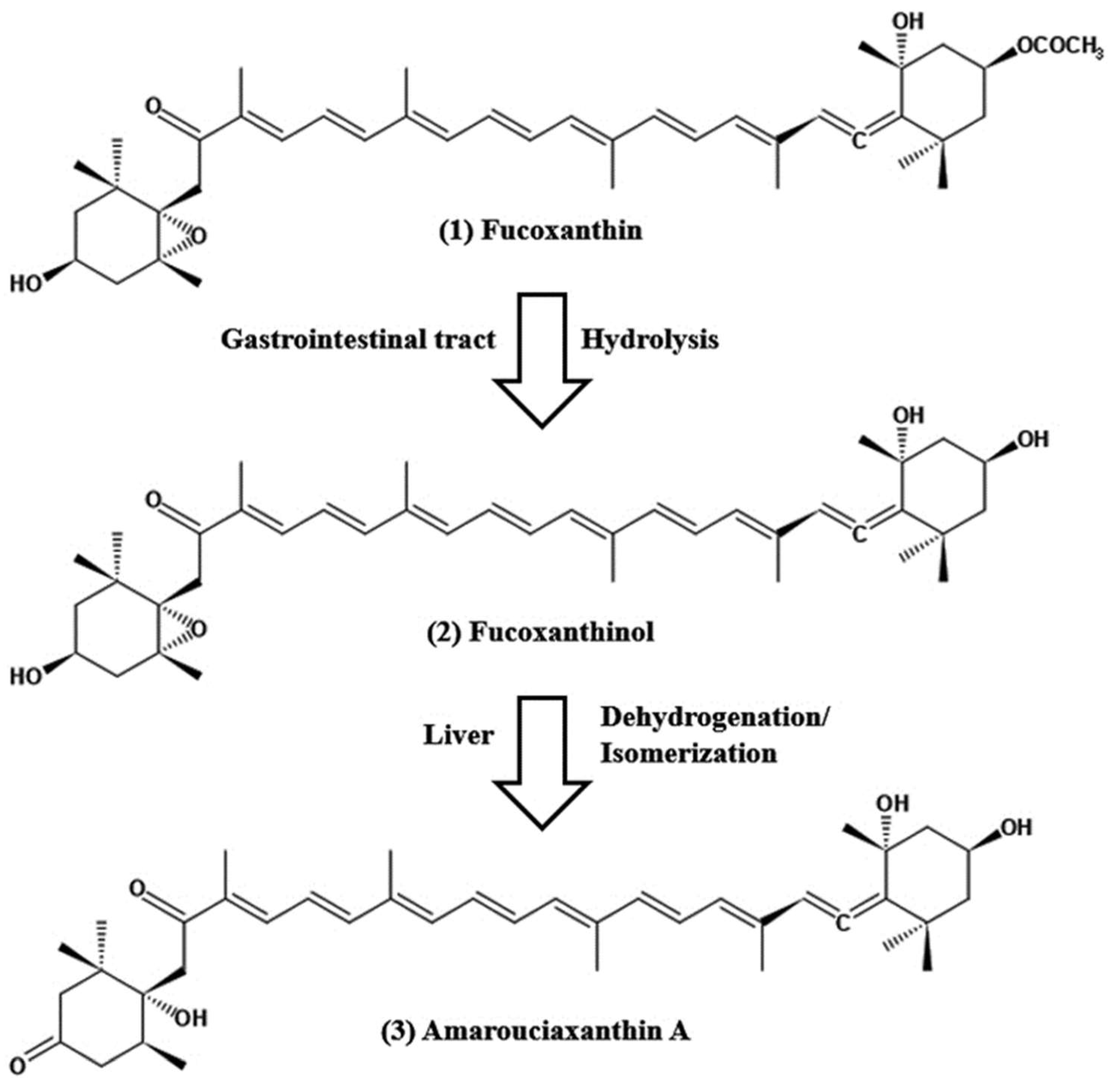
Figure 1. Chemical structure of fucoxanthin and its derivatives, fucoxanthinol by hydrolysis with digestive enzymes, lipase and cholesterol esterase in the gastrointestinal tract and ama-rouciaxanthin A by short chain dehydrogenase/reductase in the liver.
2. Pharmacokinetics of Fucoxanthin
The pharmacokinetics of fucoxanthin aims to show how a drug changes after administration through absorption, distribution, metabolism, and excretion (ADME). The absorption capability of fucoxanthin isolated from Laminaria japonica was elucidated after the peroral administration of 160 nmol per mice by Barkia et al. [3]; fucoxanthinol and amarouciaxanthin A were available after 1 h in blood plasma of which Tmax of both metabolites was 4 h and Cmax for fucoxanthinol was twice as high as amarouciaxanthin A. The metabolism of fucoxanthin (2 mg/kg) to fucoxanthinol was reported to be quicker and found after 5 min post-injection; area under the curve (AUC) of fucoxanthin was 2.5 times higher when compared with fucoxanthinol. Another study conducted after the peroral administration of 65 mg/kg; the quick conversion of fucoxanthin to fucoxanthinol was detected and found in plasma after 0.5 h of administration, Tmax values for fucoxanthin and fucoxanthinol were 7.7 and 11 h, respectively [4]. In studies on the tissue distribution of fucoxanthin and its two metabolites after peroral administration, maximal levels of fucoxanthinol and amrouciaxanthin A were reported in the liver, followed by lung, kidney, heart and spleen, and can be detected in adipose tissue until 72 h after administration, but not more than 24 h for other tissues [5]. The mean distribution volume of fucoxanthin in the blood was lower than that of its metabolite fucoxanthinol (0.7 L/kg versus 8.8 L/kg) after i.v. injection in rats [4]. Fucoxanthin metabolism is accompanied by hydrolysis with digestive enzymes, lipase, and cholesterol esterase in the gastrointestinal tract, resulting in a conversion of fucoxanthinol, which is eventually absorbed in the intestine (Figure 1). Then, it is further converted to amarouciaxanthin A in the liver by short-chain dehydrogenase/reductase (Figure 1) [6]. The conversion of fucoxanthin to fucoxanthinol was quicker when observed in both i.v. and peroral treatment in rats. The elimination of fucoxanthin was 30-fold higher than its metabolites, with fucoxanthinol (T1/2 = 4.5 h) and amarouciaxanthin A (T1/2 = 6.7 h) after peroral administration of fucoxanthin in mice [5]. Moreover, both metabolites were eliminated quickly from the liver (T1/2 = 2.5 h); however, it was much slower in the kidney (T1/2= 6.3 and 10.1 for fucoxanthinol and amarouciaxanthin A, respectively). The findings of fucoxanthin and its metabolites for pharmacokinetics supported the synergistic biofunctional activities and can be introduced as a potent drug for pharmaceuticals.
3. Safety, Toxicity, and Functional Stability of Fucoxanthin
Fucoxanthin is a naturally occurring carotenoid of brown seaweed and some microalgae which have traditionally been used as popular dietary supplements in East Asian countries for generations. This long traditional usage supports the safety of fucoxanthin upon consumption. Beppu et al. [7] conducted a toxicity study with fucoxanthin purified from seaweed in male and female ICR mice given orally at single dose concentrations of 1000 and 2000 mg/kg. In a subsequent repeat-dose study, 500 and 1000 mg/kg were administered orally for 30 days. Both studies showed no mortalities and abnormalities in the histological observation of different organs; however, total plasma cholesterol was elevated, possibly due to the interference of fucoxanthinol of the fucoxanthin metabolite or species variance that warrants further elucidation. Similar findings were reproduced in rats at doses of 10 and 50 mg/kg/day for 28 days [8]. The safety upon administration of fucoxanthin was characterized by the Ames test, micronucleus assay, and oral toxicity studies in mice and rats, showing no mutagenicity (≤5000 mg/plate), genotoxicity (≤2000 mg/plate), mortality (LD50 > 2000 mg/kg), or abnormalities in appearance of any internal organs (NOAEL > 200 mg/kg/day) [9]. Furthermore, fucoxanthinol did not cause any toxicity or adverse effects in the in vivo experiments, and can be available in human plasma after the ingestion of brown seaweed, especially Undaria pinnatifida. Studies on various models suggest that fucoxanthin can be considered a safe pharmacological ingredient [10].
The stability study of fucoxanthin is crucial to ensuring its bio-accessibility and bio-functionality in pharmaceutical development. Factors affecting the degradation of fucoxanthin include high temperature, oxygen, light, heavy metals exposure, enzymatic reactions, and long-term storage [11]. Different studies have been conducted to test the stability of fucoxanthin in terms of free molecule, emulsions, encapsulation and fucoxanthin coated nanoparticle conditions [12]. The chemical behavior of fucoxanthin was evaluated using the in vitro digestibility model, where it was exposed to different molecules with varying pH ranges from 2.2 to 7. It was found that fucoxanthin degraded progressively from simulated stomach condition (10%) and to the ileum (20%) accompanied by a transformation into fucoxanthinol. Considering that fucoxanthin is well-stabilized, the inclusion of emulsifiers could be a promising option to efficiently preserve its therapeutic potentials and chemical features over the storage period. Different emulsifiers were tested for stability, of which whey protein was the best to provide fucoxanthin with better stability during storage, as reported by Ma et al. [13]. However, further studies are needed to characterize fucoxanthin bioavailability using different emulsifiers with carrier oils. Another approach to stabilizing fucoxanthin is the encapsulation technique to prevent the alteration of core chemical structure using various microcapsules such as maltodextrin, hydroxypropyl-β-cyclodextrin, gum arabic, isolated pea protein, whey protein isolate, and gelatin [11]. A study conducted with fucoxanthin encapsulated into casein nanoparticles, and the same but with coated chitosan, was subjected to an in vitro assay of a simulated digestion process, representing its exposure to various enzymes and secreted fluids; nevertheless, nanoparticles containing fucoxanthin successfully transform fucoxanthinol through the gastrointestinal tract [12]. A recent study showed that fucoxanthin@polyvinylpyrrolidone nanoparticles effectively delivered fucoxanthin into human colon cancer cells in vitro, confirming a new and targeted therapeutic approach [14]. However, nano-particle-based fucoxanthin treatment should be introduced in animal models before further translation into clinical trials to evaluate the extent of bioactivity.
4. Clinical Perspectives of Fucoxanthin
Many of the aforementioned in vitro and in vivo studies have become reality when conducting clinical trials. As such, following the NIH ClinicalTrial.gov database, five clinical trials using either single fucoxanthin or fucoxanthin-rich extract are currently underway at different phases of trials or considering future enrolling. Moreover, a study on human subjects has reached its end, demonstrating anti-obesity effects in obese individuals administering 3 mg of fucoxanthin capsule, with a good safety profile, confirming its potential use as a pharmaceutical drug [15]. Studies with a clinical stage on the mitigation of liver health (ClinicalTrials.gov identifier: NCT03625284), the improvement of non-alcoholic fatty liver disease (ClinicalTrials.gov identifier: NCT02875392), metabolic syndrome of insulin sensitivity and secretion (ClinicalTrials.gov identifier: NCT03613740; phase 2), body weight management for overweight women (ClinicalTrials.gov identifier: NCT04761406), etc., have yet to publish their results. Moreover, cognitive decline in aged populations is a major challenge in the coming years. With mounting evidence of in vitro and in vivo experiments in the recent past, a clinical study on the neuroprotective potential of fucoxanthin-rich microalgae has been initiated since 2021 and continued to date for the validation of its efficacy for future pharmaceutical application (ClinicalTrials.gov identifier: NCT04832412). Considering recent studies, fucoxanthin can be introduced as a lead drug with multi-targets or a pro-type drug for future pharmaceutical or nutraceutical development.
5. Pharmaceutical Prospects of Fucoxanthin
Fucoxanthin is a naturally occurring potent antioxidant molecule with noteworthy and diverse bioactivities. The growing body of scientific in vitro and in vivo evidence leads us to understand the maximization of its utilization in viable and systematic methods for nutraceutical and pharmaceutical development. Since 2017, studies on fucoxanthin have mostly reported promising antioxidant, anti-inflammatory, and anti-apoptotic potentials, together these effects synergistically protect against cancer, neurodegeneration, hyperlipidemia, diabetics, cardiac, liver, kidney, eye and skin diseases, among others. Figure 2 shows the various pharmacological properties of fucoxanthin with possible molecular actions mechanisms by targeting multiple signaling pathways. These effects turn fucoxanthin into further clinical research to evaluate its efficacy in each disease condition before developing it as a therapeutic drug.
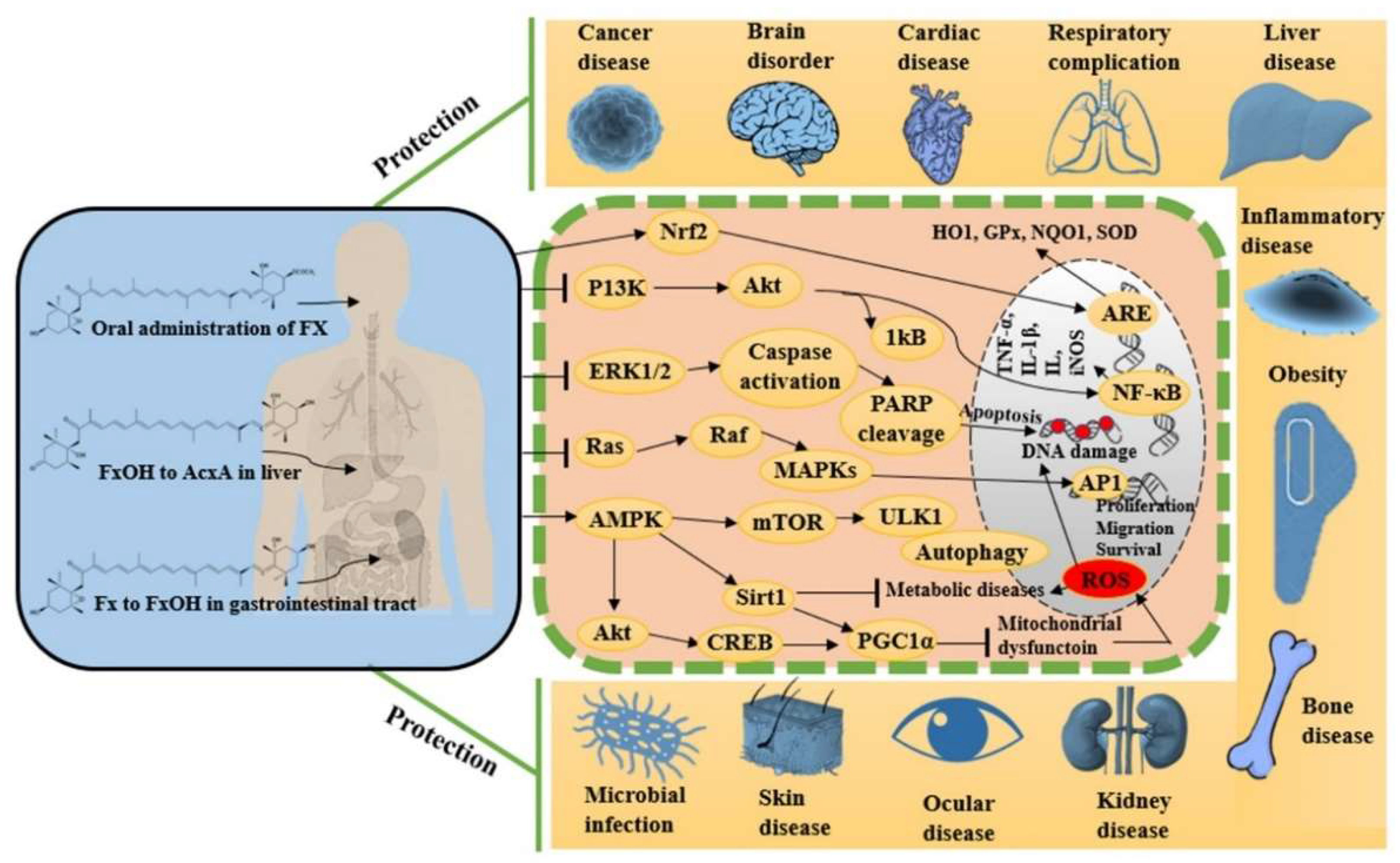
Figure 2. Metabolism of fucoxanthin and its derivatives after administration and their pharmacological modulation in various cellular pathways.
Fucoxanthin extracted from edible brown seaweed commercially has been suggested for pharmaceutical or medicinal uses, as it is safe and has no toxicity upon administration. However, the availability of fucoxanthin for industrial uses, unfortunately, is limited, and a chemical process for fucoxanthin synthesis is complex and eventually expensive for endpoint users. To scale up the industrial production, an effective, easy, and low-cost solvent extraction method should be introduced, and standardized to maximize its yield. Thus, seaweed with the highest fucoxanthin content requires more attention to intensive culture rather than depending on natural sources to ensure its year-round availability. The post-extraction stability studies of fucoxanthin recommend that its use at the industry level should be kept at a low temperature and free from heat and light as well, because it is vulnerable to all of these extrinsic factors. Some studies suggest using it in an encapsulated form with either emulsifiers or nanoparticles to preserve its bio-accessibility and bio-functionality while taken as food ingredients or pharmaceutical drugs. During the oral administration of fucoxanthin, two of its metabolites, fucoxanthinol, and amarouciaxanthin A, are readily accessible to the blood plasma, and are located in various organs such as the liver, kidney, and heart for bio-functionalities; those studies were conducted in vivo and human trials [10][11][13]. A wide array of health functional properties of fucoxanthin has been relevant for its future applications both in food supplementation as an active ingredient and pharmaceutical industries as a potent drug.
This entry is adapted from the peer-reviewed paper 10.3390/md20050279
References
- Aman, R.; Schieber, A.; Carle, R. Effects of heating and illumination on trans-cis isomerization and degradation of beta-carotene and lutein in isolated spinach chloroplasts. J. Agric. Food Chem. 2005, 53, 9512–9518.
- Kawee-ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat. Prod. Commun. 2013, 8, 1381–1386.
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304.
- Zhang, Y.; Wu, H.; Wen, H.; Fang, H.; Hong, Z.; Yi, R.; Liu, R. Simultaneous Determination of Fucoxanthin and Its Deacetylated Metabolite Fucoxanthinol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 6521–6536.
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248.
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557.
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510.
- Kadekaru, T. Safety evaluation of fucoxanthin purified from Undaria pinnatifida. Nippon Shokuhin Kagaku Kogaku Kaishi 2008, 55, 304–308.
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828.
- Miyashita, K.; Hosokawa, M.J. Fucoxanthin in the management of obesity and its related disorders. J. Funct. Foods 2017, 36, 195–202.
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113.
- Koo, S.Y.; Mok, I.K.; Pan, C.H.; Kim, S.M. Preparation of Fucoxanthin-Loaded Nanoparticles Composed of Casein and Chitosan with Improved Fucoxanthin Bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435.
- Ma, Z.; Khalid, N.; Shu, G.; Zhao, Y.; Kobayashi, I.; Neves, M.A.; Tuwo, A.; Nakajima, M. Fucoxanthin-Loaded Oil-in-Water Emulsion-Based Delivery Systems: Effects of Natural Emulsifiers on the Formulation, Stability, and Bioaccessibility. ACS Omega 2019, 4, 10502–10509.
- Sui, Y.; Gu, Y.; Lu, Y.; Yu, C.; Zheng, J.; Qi, H. nanoparticles promoted oxidative stress-induced cell death in Caco-2 human colon cancer cells. Mar. Drugs 2021, 19, 92.
- Hitoe, S.; Shimoda, H. Seaweed fucoxanthin supplementation improves obesity parameters in mild obese Japanese subjects. Funct. Foods Health Dis. 2017, 7, 246–262.
This entry is offline, you can click here to edit this entry!
