Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Neuroinflammation has recently been identified as a fundamentally important pathological process in most, if not all, CNS diseases. The main contributor to neuroinflammation is the microglia, which constitute the innate immune response system. Accurate identification of microglia and their reactivity state is therefore essential to further our understanding of CNS pathophysiology. Many staining techniques have been used to visualise microglia in rodent and human tissue, and immunostaining is currently the most frequently used.
- microglia
- neuroinflammation
- immunostaining
1. Introduction
Neuroinflammation has recently been identified as a crucially important pathological process in most if not all central nervous system (CNS) diseases. Since the main contributor of neuroinflammation is the microglia that modulate the innate immune response, an understanding of their pathophysiological roles in CNS disease will likely be essential to the discovery of effective therapies [1]. However, it has previously been difficult to clearly distinguish microglia from meningeal, perivascular, choroid plexus, and circulating macrophages in pathological conditions [2].
The origin of microglia was for a long time though to derive from blood monocytes in the bone marrow, however in 1999 Alliot et al. showed that microglia were in fact derived from progenitors originating in the yolk sac [3]. This was further confirmed using fate-mapping analysis that showed that microglia migrate from the embryonic yolk sac at approximately E9.5 in mice and 4.5 weeks gestation in humans [4]. After the microglia replicate, they spread throughout the whole CNS in a cellular grid formation until adulthood, eventually comprising approximately 10% of the total glial cell population [5,6].
Currently, there is a growing body of literature that recognises the importance of microglia and their functional roles in CNS homeostasis and pathophysiology. During homeostasis in the adult, microglia play several key roles: the long and thin branching processes provide constant surveillance of the local environment to maintain tissue integrity, such as repairing the blood vessels [7,8], and permitting synaptic plasticity by engulfing synapses [9]. Furthermore, connections between perivascular microglia and endothelial cells, along with pericytes and astrocytes, form the neurovascular unit that maintains blood-brain barrier integrity [10].
Microglia have been implicated as a crucial player in disease pathophysiology. In their normal state, the ramified (‘branch like’)/resting (non-reactive phenotype) microglia exhibit a morphology of very thin and long processes with a small cell body (Figure 1A–C, Figure 2A–C, Figure 3A–C, Figure 4A–C, Figure 5A–C and Figure 6A–C, unpublished data). In pathological conditions, the microglia undergo a transition that retracts their processes resulting in thicker and shorter processes and a larger cell body, until finally the processes are significantly reduced, which is termed amoeboid (‘round like’)/reactive microglia (Figure 1D–O, Figure 2D–O, Figure 3D–O, Figure 4D–O, Figure 5D–O and Figure 6D–O, unpublished data). Interestingly, in the examples of microglial activation states provided in this entry (all seen within a 1.5 mm cross-section of a brain biopsy from a patient suffering severe traumatic brain injury (TBI)), it is possible to see both ramified and various other reactive states of microglia. This suggests that the microglial response is at a cellular level rather than a global cellular response to TBI.
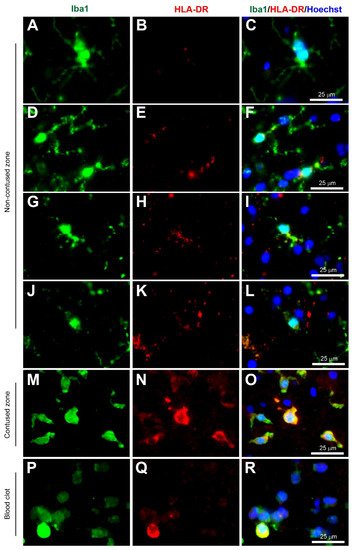
Figure 1. Coexpression of Iba1 and HLA-DR. Human brain biopsy samples from the superior frontal gyrus after TBI were stained with the primary antibodies: rabbit anti-Iba1 (1:1000, Wako, Cat No. 019-19741) and mouse anti-HLA-DR (1:500, Invitrogen, Cat No. MA5-11966) followed by rabbit anti-Alexa Fluor 488 and mouse anti-Alexa Fluor 594, respectively. Limited HLA-DR expression was observed in Iba1+ microglia with a ramified morphology (A–C), but strong HLA-DR expression in Iba1+ microglia in the activated state (D–L) in the non-contused brain tissue. Predominantly amoeboid microglia stained with Iba1 and HLA-DR were observed in contused brain tissue (M–O). Clotted blood from a neurosurgical (brain) operative field was used to identify a few peripherally-derived macrophages that strongly stained for both Iba1 and HLA-DR (P–R). Scale bars are 25 μm. Unpublished data.
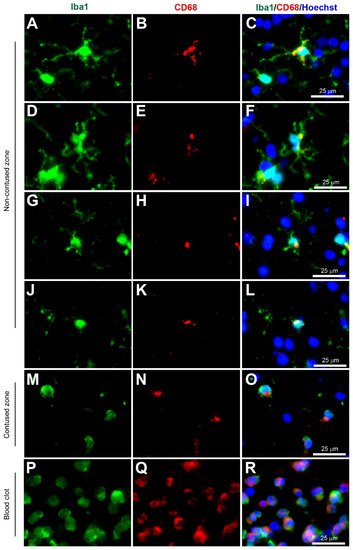
Figure 2. Coexpression of Iba1 and CD68. Human brain biopsy samples from the superior frontal gyrus after TBI were stained with the primary antibodies: rabbit anti-Iba1 (1:1000, Wako, Cat No. 019-19741) and mouse anti-CD68 (1:500, Invitrogen, Cat No. 14-0688-82) followed by rabbit anti-Alexa Fluor 488 and mouse anti-Alexa Fluor 594, respectively. Iba1+ microglia co-expressed with CD68 can be observed in various activated states, ranging from the most ramified state (A–C) through increasing states of activation (D–F and G–I) to the most amoeboid morphology (J–L) in the non-contused brain tissue. In contrast, predominantly amoeboid microglia stained with Iba1 and CD68 were observed in contused brain tissue (M–O). Clotted blood from a neurosurgical (brain) operative field was used to identify peripherally-derived macrophages that strongly stained for both Iba1 and CD68 (P–R). Scale bars are 25 μm. Unpublished data.
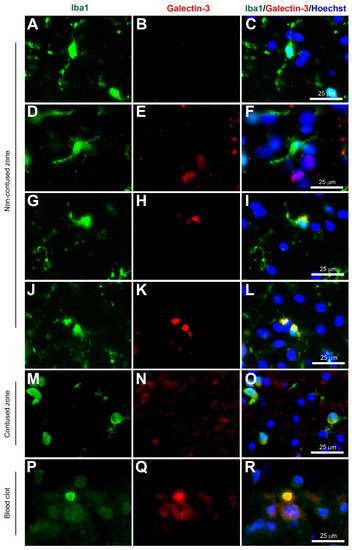
Figure 3. Coexpression of Iba1 and galectin-3. Human brain biopsy samples from the superior frontal gyrus after TBI were stained with the primary antibodies: rabbit anti-Iba1 (1:1000, Wako, Cat No. 019-19741) and goat anti-galectin-3 (1:500, BioLegend, Cat No. 126701) followed by rabbit anti-Alexa Fluor 488 and goat anti-Alexa Fluor 594, respectively. Limited galectin-3 expression was observed in Iba1+ microglia with a ramified morphology (A–C), but there was a strong galectin-3 expression in Iba1+ microglia in the activated state (D–L) in the non-contused brain tissue. Predominantly amoeboid microglia stained with Iba1 and galectin-3 were observed in contused brain tissue (M–O). Clotted blood from a neurosurgical (brain) operative field was used to identify peripherally-derived macrophages that strongly stained for both Iba1 and galectin-3 (P–R). Scale bars are 25 μm. Unpublished data.
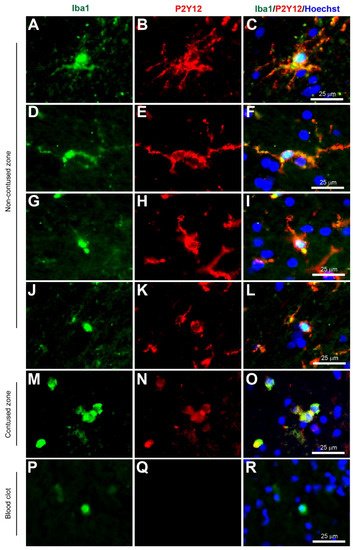
Figure 4. Coexpression of Iba1 and P2Y12. Human brain biopsy samples from the superior frontal gyrus after TBI were stained with the primary antibodies: goat anti-Iba1 (1:500, Novus Biologicals, Cat No. NB100-1028) and rabbit anti-P2Y12 (1:200, AnaSpec, ANA55043A) followed by goat anti-Alexa Fluor 488 and rabbit anti-Alexa Fluor 594, respectively. Strong coexpression was observed in Iba1+ microglia with P2Y12 in both ramified (A–C) and activated state (D–L) in the non-contused brain tissue. Predominantly amoeboid microglia stained with Iba1 and P2Y12 were observed in contused brain tissue (M–O). Clotted blood from a neurosurgical (brain) operative field was used to identify peripherally-derived macrophages that showed strong Iba1+ but lacked P2Y12+ immunostaining (P–R). Scale bars are 25 μm. Unpublished data.
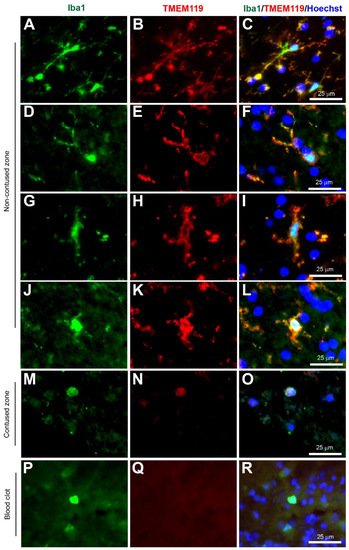
Figure 5. Coexpression of Iba1 and TMEM119. Human brain biopsy samples from the superior frontal gyrus after TBI were stained with the primary antibodies: goat anti-Iba1 (1:500, Novus Biologicals, Cat No. NB100-1028) and rabbit anti-TMEM119 (1:500, Abcam, Cat No. ab185333) followed by goat anti-Alexa Fluor 488 and rabbit anti-Alexa Fluor 594, respectively. Strong coexpression was observed in Iba1+ microglia with TMEM119 in both ramified (A–C) and activated state (D–L) in the non-contused brain tissue. Predominantly amoeboid microglia stained with Iba1 and TMEM119 were observed in contused brain tissue (M–O). Clotted blood from a neurosurgical (brain) operative field was used to identify peripherally-derived macrophages that showed strong Iba1+ but lacked TMEM119+ immunostaining (P–R). Scale bars are 25 μm. Unpublished data.
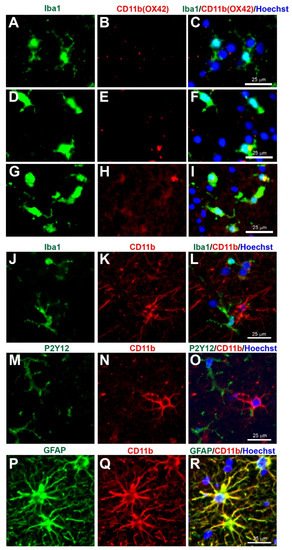
Figure 6. Concerns with CD11b (Clone OX42) immunostaining for microglia in human tissue. Human brain biopsy samples from the superior frontal gyrus after TBI were stained with the primary antibodies: mouse anti-rat OX42 (1:100, Bio_Rad, Cat. No. MCA275R) (A–I), or mouse anti-human CD11b (1:400, Novus Biologicals, Cat. No. MAB16991) (J–R); then with rabbit anti-Iba1 (1:1000, Wako, Cat No. 019-19741) (J), or rabbit anti-P2Y12 (1:200, AnaSpec, ANA55043A) (M), or rabbit anti-GFAP (1:1000, Dako, Cat No. Z0334) (P), followed by rabbit anti-Alexa Fluor 488 and mouse anti-Alexa Fluor 594 or tyramide amplification with ExtrAvidin FITC for the mouse anti-human CD11b (K,N,Q). Limited anti-rat OX42 expression was observed in Iba1+ microglia with a ramified state morphology (A–C), but some anti-rat OX42 expression in Iba1+ microglia in the more activated state (D–F) or contused brain tissue (G–I). Anti-human CD11b staining was absent in Iba1 (J–L) and P2Y12 (M–O) immunopositive cells, but coexpressed with the GFAP astrocytic marker (P–R). Scale bars are 25 μm. Unpublished data.
Microglia are thought to be significant contributors to various CNS diseases by releasing cytokines and by displaying phagocytic properties. In amyotrophic lateral sclerosis, there is evidence to support both neuroprotective roles and also neurotoxicity [11]. Impaired microglial activity and altered microglial response to amyloid beta protein are associated with an increased risk of Alzheimer’s disease. However, there is also abundant evidence that activated microglia can be harmful to neurones [12]. In TBI, dysregulation of microglia can release high levels of pro-inflammatory and cytotoxic mediators that contribute to neuronal dysfunction and cell death. However, microglia in the injured brain can also produce neuroprotective factors, clear cellular debris, and orchestrate neurorestorative processes that are beneficial for neurological recovery after TBI [13]. Interestingly, in spinal cord injury, recent evidence suggests that microglia are essential to the formation of the neuroprotective glial scar [14], and that microglial depletion exacerbates injury and impairs functional recovery [15]. Although microglia are deleterious in ischemic stroke, there is also evidence to suggest that microglial activation is critical for promoting functional recovery through neurogenesis, angiogenesis, and synaptic remodeling [16]. Overall, the roles of microglia in CNS injuries are extremely complex and require further investigation.
Histological staining is one of the most common techniques for the identification of any cell type, including microglia, as opposed to Western blotting or enzyme-linked immunosorbent assay (ELISA). Immunohistochemistry staining is a fairly simple procedure to perform, identifies the exact cellular location within the CNS, provides a morphological representation of cellular processes, and simultaneously expresses the intensity level of one to several proteins of interest. Furthermore, it is highly sensitive and selective when a particular immunostaining method is used. In contrast, Western blotting or ELISA (for example) generates a single signal proportional to the amount of protein present in a homogenised tissue sample.
2. History of Microglial Staining
Research in this field was pioneered by Franz Nissl, Santiago Ramón y Cajal, and Pío Del Río Hortega, with the latter scientist rewarded with the ‘father of microglia’ accolade [18]. During the time of the first microglia discovery, different methods of impregnation of brain tissue with various stains to identify cells in nervous tissue were used [19]. In 1899, Franz Nissl, using his eponymous Nissl stain, was the first to identify cells he termed stäbchenzellen that were rod-like microglial cells, but he did not pursue this further [20]. It was not until 1913 that the origins of microglia were revealed when Ramón Y Cajal used a silver chromate staining technique to identify a group of cells that were non-neuronal and non-astrocytic in morphology [21]. However, it was Pío Del Río Hortega who in 1919, used a modified silver carbonate impregnation method that stained a selective group of glial cells, and named one of these new populations of cells microglia [8,22]. He also introduced the concept of plasticity in microglia after discovering that they could change their morphological shape depending on the brain’s pathological state.
Identification of microglia is facilitated by the use of appropriate staining techniques, and the suitability of a stain is based on its ability to discriminate microglia from other CNS cell populations including neurons, astrocytes, endothelial cells, and macrophages. Furthermore, stains must have sufficient ability to stain either all microglia, or only certain activation states [8]. Del Río-Hortega’s ammoniacal silver carbonate method not only stains microglia but also oligodendrocytes, and it is a complex protocol and requires optimum conditions for success [23]. Therefore a more selective, quicker, and easier staining protocol is required. Furthermore, relying on cell morphology can be problematic since microglia transform into different morphological shapes depending on their activation state.
3. Microglia Staining Development
Albert Coons and colleagues were the first to demonstrate selective staining through antigen-antibody interactions using an antibody and fluorescent dye [24]. Thereafter, other immunohistochemical techniques using enzyme labels such as peroxidase and alkaline phosphatase were developed [25]. This revolutionised the histological field by offering a more detailed spatiotemporal expression of specific antigens, apart from solely relying on morphology. Since the mid-1900s, researchers have tried many various markers ranging from ATPase to the recent TMEM119 to selectively identify microglia (Table 1).
3.1. ATPase
Chronologically, the Adenosine triphosphatase (ATPase) stain was first used in the identification of microglial cells beyond Hortega’s silver chloride method. The enzymatic marker ATPase was discovered in the 1970s to be among the most selective enzymatic markers for microglia in rat and rabbit brain tissues, obtained from the normal brain and after a stab wound injury [26]. Although the cytochemical staining protocol using the Wachstein-Meisel medium was successfully used to identify microglia, ATPase was also located in various other cellular regions such as vessel walls, astrocytes, oligodendrocytes, and proximal axons [26]. This is not surprising as ATPase is an essential enzyme for energy, active transport, and pH homeostasis in all cell types [27,28]. Furthermore, other studies that used this method demonstrated the presence of ATPase in rat placenta, kidney, heart, prostate, uterus, and leg muscle [29]. Overall, these findings suggested that ATPase staining cannot be used as a specific marker for microglia.
3.2. Iba1
The Ionized calcium-binding adaptor molecule 1 (Iba1) immunostain was a significant breakthrough in terms of imaging and identification of microglial cells. Iba1 is located in a major histocompatibility complex (MHC) class III region, encoding an EF-hand protein [30]. A research study in 1998 suggested this novel calcium-binding protein Iba-1 was a microglia-specific marker [31]. Moreover, Iba1+ immunostaining can clearly label the cell body and the fine processes in ramified microglia, and that reactive microglia express greater Iba1+ levels compared to non-reactive ramified microglia, and it was therefore less dependent on the morphological shape of ramified or amoeboid [31] (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6, unpublished data). Since this discovery, the majority of research papers with histological analysis of microglia included the use of Iba1 immunostaining to observe Iba1-positive cells [32]. Although Ito and colleagues suggested the Iba1 marker was microglia-specific, research has suggested that Iba1 is also an optimum marker for macrophages in both rats and humans [33,34]. Since reactive microglia and macrophages exhibit similar phenotypes, it is reasonable to assume that they express similar molecules. Overall, Iba1 can selectively distinguish from other glial cells within the CNS, but it is unable to distinguish between microglia and macrophages as both have high Iba1 expression, so care is required when interpreting Iba1+ immunostained cells in human post-mortem and/or animal tissue. However, most highly reactive microglia will exhibit some form of extrusion in comparison to the spherical amoeboid-shaped macrophages (Figure 1M−R, Figure 2M−R, Figure 3M−R, Figure 4M−R, Figure 5M−R and Figure 6M−R, unpublished data) [35]. Therefore, using morphological differences (i.e., microglia exhibit processes and macrophages do not exhibit processes) in combination with location within the tissue (i.e., microglia are within the intact CNS tissue and macrophages are on the periphery or within vasculature) affords the possibility of using Iba1 to stain for microglia within the CNS.
3.3. HLA-DR
Another MHC-related microglia marker is the Human Leukocyte Antigen—DR isotype (HLA-DR). This glycoprotein complex is an antigen-presenting molecule that was initially found as an MHC class II cell surface receptor on antigen-presenting cells such as macrophages, dendritic cells, and B-lymphocytes, so immunostaining is predominantly outer membrane labeling [36]. It was present on activated microglia in human Parkinson’s disease post mortem brain tissue, based on the morphology originally described by Del Rio Hortega and Penfield [37], however, McGeer and colleagues were careful to mention the HLA-DR+ staining observed in the reactive microglia, and also in brain macrophages, in the discussion. Interestingly, Gehrmann and colleagues demonstrated HLA-DR was strongly present in resting/ramified microglia, especially in the white matter [38], however, careful observation of the images of putative ramified microglia, immunopositive for HLA-DR, reveals slightly reactive microglia based on the shorter and thicker processes, in comparison to the long and thin processes observed in true ramified microglia found in normal conditions. In this entry, co-immunostaining with Iba1 (Wako, Alpha Laboratories Ltd, Cat. No. 019-19741) and HLA-DR (Invitrogen, ThermoFisher, Horsham, UK, Cat. No. MA5-11966) showed low HLA-DR+ immunostaining in ramified microglia (Figure 2A–C), but increased immunostaining in reactive microglia (Figure 2D–O) and in macrophages (Figure 2P–R) (unpublished data). Overall, HLA-DR can identify reactive microglia if the microglia exhibit processes, however, it is difficult to distinguish them from other peripheral immune cells such as monocytes-derived macrophages when in the full reactive state.
This entry is adapted from the peer-reviewed paper 10.3390/biom12050603
This entry is offline, you can click here to edit this entry!
