Scholars have recently used “motor imagery” (MI) to refer to the imagination of moving particular body parts. Among the frameworks trying to explain the essence of motor imagery, there are two primary ones—motor simulation and motor emulation theory.
- motor imagery
- movement imagination
- motor imagery assessment
1. Introduction
The Motor Simulation Theory (MST) [1][2] provides a constructive explanation of the relation of imagery tasks such as motor imagery (MI task), observation, and the intention of motor tasks to motor execution (ME) tasks itself. According to MST, motor images have the same properties as analogous motor representations and thus have the same functional relationship to the perceived or represented movement and the same causal role in its development [1]. Alternatively, according to emulation theory, to simulate mechanical movement, proprioception, and kinesthesis, the “forward model” is represented by motor commands that drive body/environment emulators, which are motor and sensory representations. In such a way, coupled motor and sensory systems form a complex process of emulation that provides an adaptive motor control mechanism for ME. While performing MI, the motor system is coupled with afferent sensory systems, which provide the sensor feedback without input for MI [3].
2. Generalization of Brain Regions Involved in MI and ME
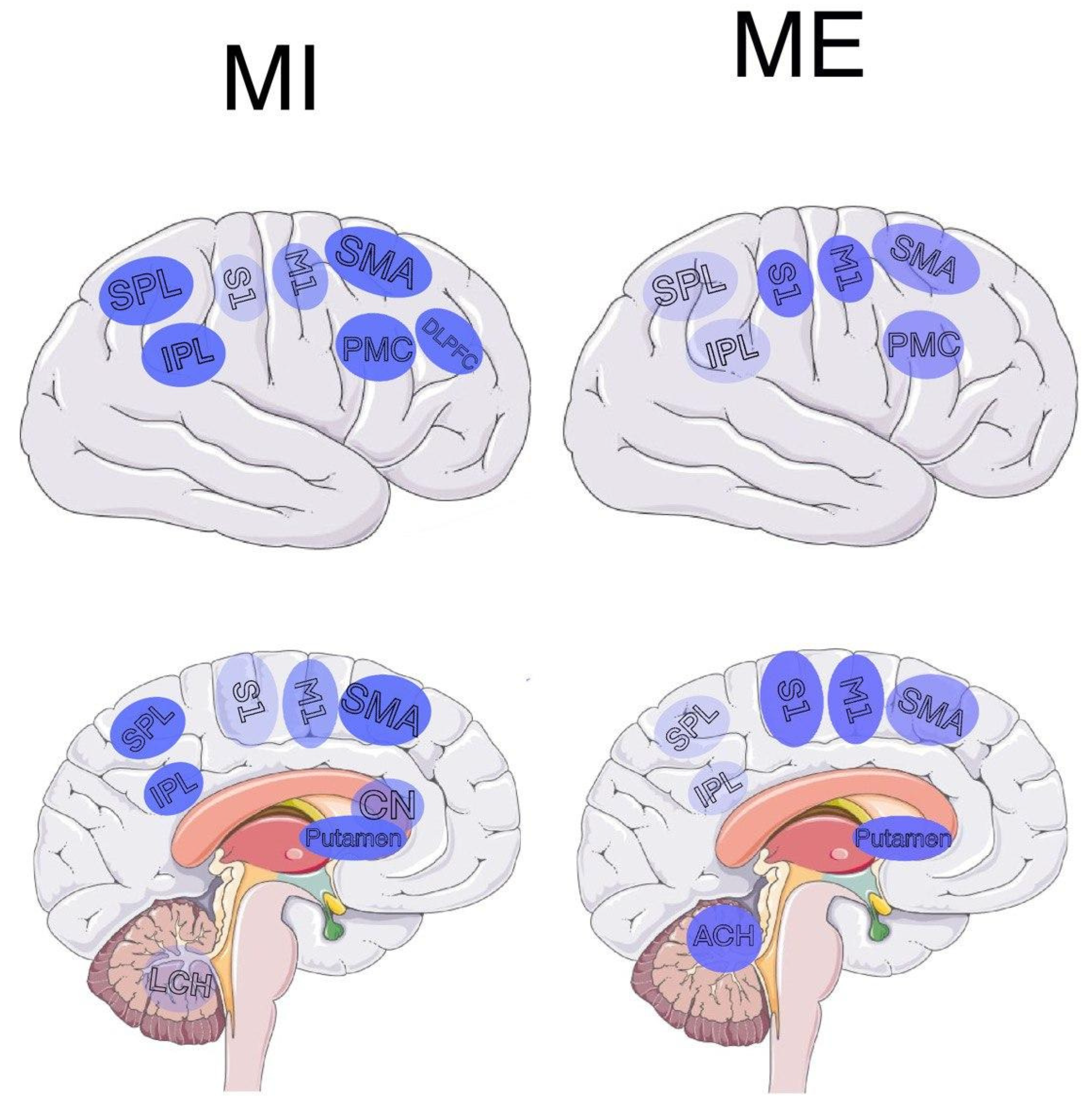
| Brain Area | Common for MI and ME | MI | ME |
|---|---|---|---|
| Inferior parietal lobe | [5][6] | [5][6][7] | [5][6] |
| Superior parietal lobe | [6][8][9] | [6][7][8][9] | [6][8][9] |
| Posterior parietal lobe | [10] | [10][11][12] | [10] |
| Frontal parietal lobe | [4][13] | [4][10][13] | [4][13] |
| Prefrontal cortex | [14] | [14] | [13][14] |
| Subcortical | [13] | [6][13] | [13] |
| Rostral premotor | - | [12][13] | - |
| Striatum | [5][13] | [5][7][13][15][16] | [5][13] |
| Cerebellar areas | [4][6][10][13][14][17] | [4][6][10][13] | [4][5][6][7][8][9][10][13][14][16][17] |
| M1 | [6][9][17][18] | [6][9][17][18][19][20] | [4][5][6][9][10][14][16][17][18][20] |
| S1 | [9][17] | [9][17] | [9][10][14][17][20] |
| S2 | [21] | [21] | [21] |
| SMA | [8][9][14][17] | [6][8][9][10][14][17] | [4][5][8][9][14][17] |
| PMC | [9][17] | [9][17] | [7][9][17] |
| Central sulcus | - | [13] | - |
| Precentral gyri | - | - | [18] |
| Frontal gyri | - | [10] | - |
| Left DLPFC | - | [22] | - |
3. Motor Imagery Assessment
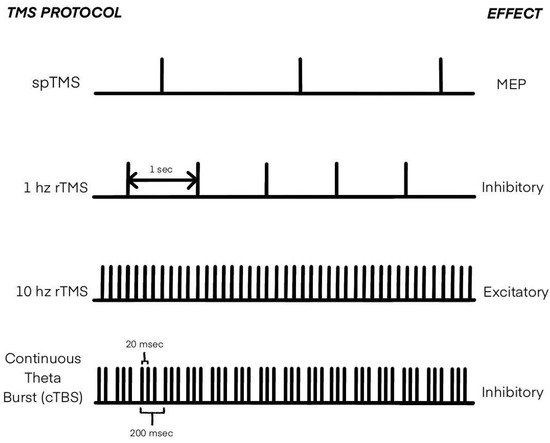
4. Transcranial Magnetic Stimulation in MI Research
5. Applications, MI for Diagnostics
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12040949
References
- Jeannerod, M. Mental imagery in the motor context. Neuropsychologia 1995, 33, 1419–1932.
- Jeannerod, M. The representing brain: Neural correlates of motor intention and imagery. Behav. Brain Sci. 2010, 17, 187–202.
- Grush, R. The emulation theory of representation: Motor control, imagery, and perception. Behav. Brain Sci. 2004, 27, 377–396.
- Hanakawa, T.; Immisch, I.; Toma, K.; Dimyan, M.A.; Van Gelderen, P.; Hallett, M. Functional properties of brain areas associated with motor execution and imagery. J. Neurophysiol. 2003, 89, 989–1002.
- Lafleur, M.F.; Jackson, P.L.; Malouin, F.; Richards, C.L.; Evans, A.C.; Doyon, J. Motor learning produces parallel dynamic functional changes during the execution and imagination of sequential foot movements. Neuroimage 2002, 16, 142–157.
- Guillot, A.; Collet, C.; Nguyen, V.A.; Malouin, F.; Richards, C.; Doyon, J. Brain activity during visual versus kinesthetic imagery: An fMRI study. Hum. Brain Mapp. 2009, 30, 2157–2172.
- Guillot, A.; Collet, C.; Nguyen, V.A.; Malouin, F.; Richards, C.; Doyon, J. Functional neuroanatomical networks associated with expertise in motor imagery. Neuroimage 2008, 41, 1471–1483.
- Nair, D.G.; Purcott, K.L.; Fuchs, A.; Steinberg, F.; Kelso, J.S. Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: A functional MRI study. Cogn. Brain Res. 2003, 15, 250–260.
- Solodkin, A.; Hlustik, P.; Chen, E.E.; Small, S.L. Fine modulation in network activation during motor execution and motor imagery. Cereb. Cortex 2004, 14, 1246–1255.
- Kuhtz-Buschbeck, J.P.; Mahnkopf, C.; Holzknecht, C.; Siebner, H.; Ulmer, S.; Jansen, O. Effector-independent representations of simple and complex imagined finger movements: A combined fMRI and TMS study. Eur. J. Neurosci. 2003, 18, 3375–3387.
- Pelgrims, B.; Andres, M.; Olivier, E. Double dissociation between motor and visual imagery in the posterior parietal cortex. Cereb. Cortex 2009, 19, 2298–2307.
- Leonardo, M.; Fieldman, J.; Sadato, N.; Campbell, G.; Ibañez, V.; Cohen, L.; Deiber, M.P.; Jezzard, P.; Pons, T.; Turner, R.; et al. A functional magnetic resonance imaging study of cortical regions associated with motor task execution and motor ideation in humans. Hum. Brain Mapp. 1995, 3, 83–92.
- Gerardin, E.; Sirigu, A.; Lehéricy, S.; Poline, J.B.; Gaymard, B.; Marsault, C.; Agid, Y.; Le Bihan, D. Partially overlapping neural networks for real and imagined hand movements. Cereb. Cortex 2000, 10, 1093–1104.
- Decety, J. Do imagined and executed actions share the same neural substrate? Cogn. Brain Res. 1996, 3, 87–93.
- Cramer, S.C.; Orr, E.L.; Cohen, M.J.; Lacourse, M.G. Effects of motor imagery training after chronic, complete spinal cord injury. Exp. Brain Res. 2007, 177, 233–242.
- Lacourse, M.G.; Orr, E.L.; Cramer, S.C.; Cohen, M.J. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage 2005, 27, 505–519.
- Lotze, M.; Montoya, P.; Erb, M.; Hülsmann, E.; Flor, H.; Klose, U.; Birbaumer, N.; Grodd, W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fMRI study. J. Cogn. Neurosci. 1999, 11, 491–501.
- Porro, C.A.; Francescato, M.P.; Cettolo, V.; Diamond, M.E.; Baraldi, P.; Zuiani, C.; Bazzocchi, M.; Di Prampero, P.E. Primary motor and sensory cortex activation during motor performance and motor imagery: A functional magnetic resonance imaging study. J. Neurosci. 1996, 16, 7688–7698.
- Lang, W.; Cheyne, D.; Höllinger, P.; Gerschlager, W.; Lindinger, G. Electric and magnetic fields of the brain accompanying internal simulation of movement. Cogn. Brain Res. 1996, 3, 125–129.
- Roth, M.; Decety, J.; Raybaudi, M.; Massarelli, R.; Delon-Martin, C.; Segebarth, C.; Morand, S.; Gemignani, A.; Décorps, M.; Jeannerod, M. Possible involvement of primary motor cortex in mentally simulated movement: A functional magnetic resonance imaging study. Neuroreport 1996, 7, 1280–1284.
- Dechent, P.; Merboldt, K.D.; Frahm, J. Is the human primary motor cortex involved in motor imagery? Cogn. Brain Res. 2004, 19, 138–144.
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44.
- Galton, F. Mental Imagery. In Inquiries into Human Faculty and Its Development; MacMillan Co.: New York, NY, USA, 1883; pp. 83–114.
- Richardson, A.; Sheikh, A.A. Individual Differences in Imaging: Their Measurement, Origins, and Consequences; Routledge: London, UK, 2020.
- Anquetil, T.; Jeannerod, M. Simulated actions in the first and in the third person perspectives share common representations. Brain Res. 2007, 1130, 125–129.
- Smyth, M.M.; Waller, A. Movement imagery in rock climbing: Patterns of interference from visual, spatial and kinaesthetic secondary tasks. Appl. Cogn. Psychol. 1998, 12, 145–157.
- Wassermann, E.; Epstein, C.; Ziemann, U.; Walsh, V. Oxford Handbook of Transcranial Stimulation; Oxford Library of Psychology, OUP Oxford: Oxford, UK, 2008.
- Mantovani, A.; Pavlicova, M.; Avery, D.; Nahas, Z.; McDonald, W.M.; Wajdik, C.D.; Holtzheimer, P.E., III; George, M.S.; Sackeim, H.A.; Lisanby, S.H. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (tms) in treatmnt-resistant depression. Depress. Anxiety 2012, 29, 883–890.
- Shindo, K.; Sugiyama, K.; Huabao, L.; Nishijima, K.; Kondo, T.; Izumi, S. Long-term effect of low-frequency repetitive transcranial magnetic stimulation over the unaffected posterior parietal cortex in patients with unilateral spatial neglect. J. Rehabil. Med. 2006, 38, 65–67.
- Hayashi, T.; Ohnishi, T.; Okabe, S.; Teramoto, N.; Nonaka, Y.; Watabe, H.; Imabayashi, E.; Ohta, Y.; Jino, H.; Ejima, N.; et al. Long-term effect of motor cortical repetitive transcranial magnetic stimulation induces. Ann. Neurol. 2004, 56, 77–85.
- Hramov, A.E.; Maksimenko, V.A.; Pisarchik, A.N. Physical principles of brain–computer interfaces and their applications for rehabilitation, robotics and control of human brain states. Phys. Rep. 2021, 918, 1–133.
- Hramov, A.E.; Grubov, V.; Badarin, A.; Maksimenko, V.A.; Pisarchik, A.N. Functional near-infrared spectroscopy for the classification of motor-related brain activity on the sensor-level. Sensors 2020, 20, 2362.
- Zimmermann-Schlatter, A.; Schuster, C.; Puhan, M.A.; Siekierka, E.; Steurer, J. Efficacy of motor imagery in post-stroke rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2008, 5, 1–10.
- Grigorev, N.A.; Savosenkov, A.O.; Lukoyanov, M.V.; Udoratina, A.; Shusharina, N.N.; Kaplan, A.Y.; Hramov, A.E.; Kazantsev, V.B.; Gordleeva, S. A BCI-based vibrotactile neurofeedback training improves motor cortical excitability during motor imagery. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1583–1592.
- Mizuguchi, N.; Nakata, H.; Uchida, Y.; Kanosue, K. Motor imagery and sport performance. J. Phys. Fit. Sports Med. 2012, 1, 103–111.
- Ladda, A.M.; Lebon, F.; Lotze, M. Using motor imagery practice for improving motor performance—A review. Brain Cogn. 2021, 150, 105705.
- Abidi, M.; De Marco, G.; Grami, F.; Termoz, N.; Couillandre, A.; Querin, G.; Bede, P.; Pradat, P.F. Neural correlates of motor imagery of gait in amyotrophic lateral sclerosis. J. Magn. Reson. Imaging 2021, 53, 223–233.
- Helmich, R.C.; de Lange, F.P.; Bloem, B.R.; Toni, I. Cerebral compensation during motor imagery in Parkinson’s disease. Neuropsychologia 2007, 45, 2201–2215.
- Lulé, D.; Diekmann, V.; Kassubek, J.; Kurt, A.; Birbaumer, N.; Ludolph, A.C.; Kraft, E. Cortical plasticity in amyotrophic lateral sclerosis: Motor imagery and function. Neurorehabil. Neural Repair 2007, 21, 518–526.
- Wang, L.; Zhang, Y.; Zhang, J.; Sang, L.; Li, P.; Yan, R.; Qiu, M.; Liu, C. Aging changes effective connectivity of motor networks during motor execution and motor imagery. Front. Aging Neurosci. 2019, 312.
- Hopulele-Petri, A.; Manea, M. Visual and motor functions in schizophrenia. Eur. Psychiatry 2017, 41, S198–S199.
- Danckert, J.; Saoud, M.; Maruff, P. Attention, motor control and motor imagery in schizophrenia: Implications for the role of the parietal cortex. Schizophr. Res. 2004, 70, 241–261.
- Lallart, E.; Jouvent, R.; Herrmann, F.R.; Beauchet, O.; Allali, G. Gait and motor imagery of gait in early schizophrenia. Psychiatry Res. 2012, 198, 366–370.
- Décombe, A.; Brunel, L.; Murday, V.; Osiurak, F.; Capdevielle, D.; Raffard, S. Getting a tool gives wings even in schizophrenia: Underestimation of tool-related effort in a motor imagery task. NPJ Schizophr. 2021, 7, 1–8.
- Liberg, B.; Adler, M.; Jonsson, T.; Landén, M.; Rahm, C.; Wahlund, L.O.; Wiberg-Kristoffersen, M.; Wahlund, B. Motor imagery in bipolar depression with slowed movement. J. Nerv. Ment. Dis. 2013, 201, 885–893.
- Bennabi, D.; Monnin, J.; Haffen, E.; Carvalho, N.; Vandel, P.; Pozzo, T.; Papaxanthis, C. Motor imagery in unipolar major depression. Front. Behav. Neurosci. 2014, 8, 413.
- Conson, M.; Mazzarella, E.; Frolli, A.; Esposito, D.; Marino, N.; Trojano, L.; Massagli, A.; Gison, G.; Aprea, N.; Grossi, D. Motor imagery in Asperger syndrome: Testing action simulation by the hand laterality task. PLoS ONE 2013, 8, e70734.
