1. Introduction
Historically, the term cell death comprised apoptosis, necrosis and autophagy, each possessing its own characteristic features, and—in case the of apoptosis and autophagy—specific molecular machineries and genetic programs
[1][2]. Among these ‘classical’ forms of cell death, apoptosis was long considered to be the only form of regulated cell death (RCD), which made it an attractive target for pharmacological intervention by anticancer drugs
[3]. In 2012, Dixon et al. described a new form of regulated cell death that functions independently of the apoptotic machinery and possesses its own morphological, biochemical and molecular characteristics
[4][5]. As this new form of RCD is dependent on iron (accumulation) and oxidative stress-induced lipid peroxidation, it was termed ferroptosis
[5][6]. Interestingly, earlier studies already found that treatment of cells with the substances erastin and RSL (which are now defined as class I and class II ferroptosis inducers) led to non-apoptotic cell death events
[7][8]; however, Dixon et al. were the first to describe the underlying process of ferroptosis
[4]. Due to the endpoint of ferroptosis characterized by a loss of plasma membrane integrity, its rupture and release of intracellular material, ferroptosis is currently considered a form of regulated necrosis
[9][10] with a necrotic (lytic) morphology including typical mitochondrial changes (such as shrinkage, reduced cristae)
[5][11].
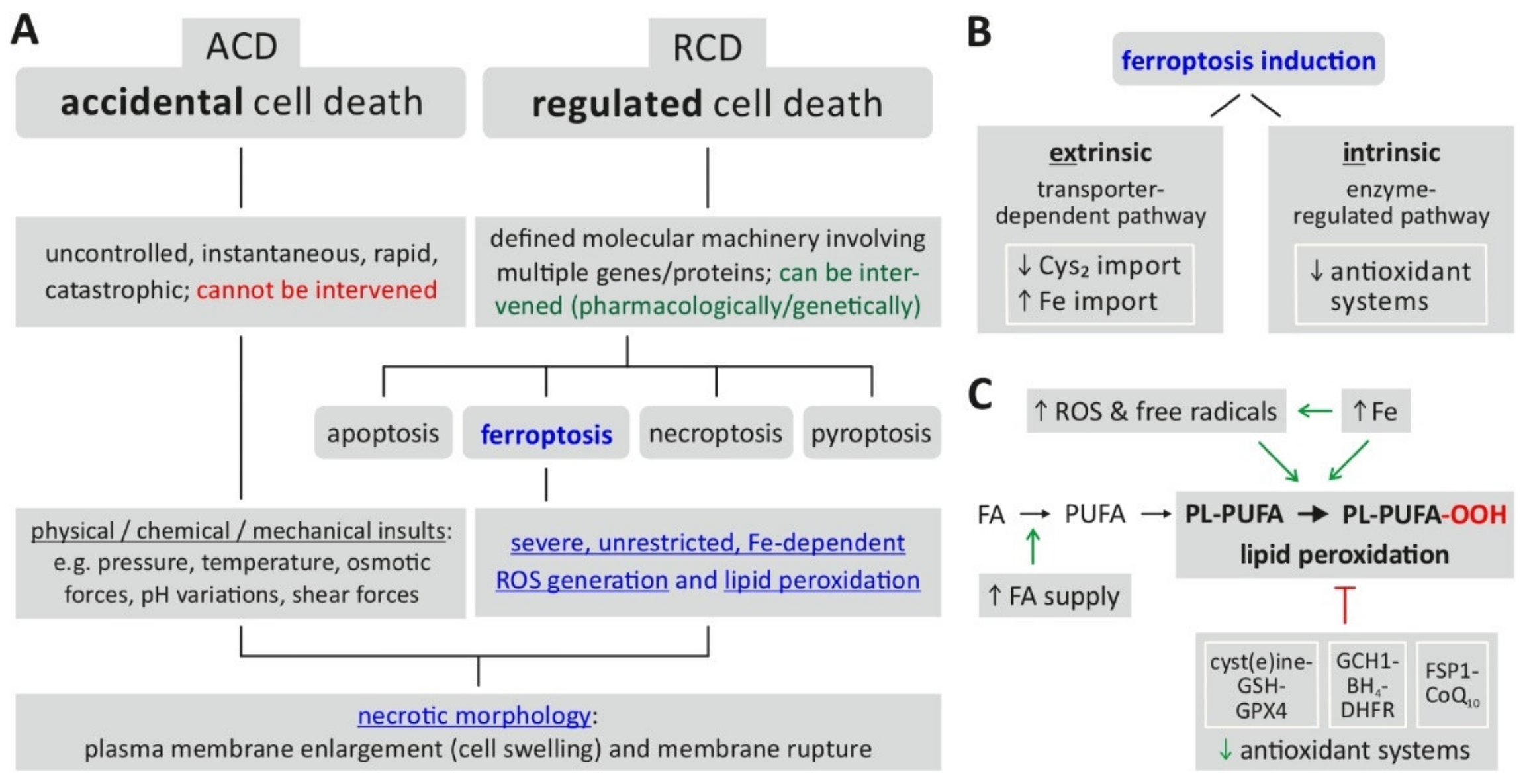
Figure 1A indicates the position of ferroptosis in the currently defined spectrum of cell death mechanisms.
Figure 1. Ferroptosis—classification, characteristics, induction. (
A) Ferroptosis is classified as a form of regulated cell death characterized by severe, uncontrolled lipid peroxidation and loss of cell membrane integrity. (
B) The prerequisites for induction of ferroptosis include the generation of reactive oxygen species (ROS) fueled by an increase of intracellular iron (Fe), which cause oxidation of membrane lipids. The changes are enabled (facilitated) by a reduced activity of cellular antioxidant defense mechanisms. (
C) Ferroptosis induction is further classified into an extrinsic or intrinsic pathway. Abbreviations: ACD = accidental cell death, BH4 = tetrahydrobiopterin, CoQ10 = coenzyme Q10, Cys2 = cystine, DHFR = dihydrofolate reductase, FA = fatty acid, FSP1 = ferroptosis suppressor protein 1, GCH1 = GTP cyclohydrolase 1, GPX4 = glutathione peroxidase 4, GSH = glutathione (reduced), PL-PUFA = PUFA-containing phospholipid, PL-PUFA-OOH = phospholipid hydroperoxide, PUFA = polyunsaturated fatty acid, RCD = regulated cell death, ROS = reactive oxygen species. Based on:
[5][6][10][12].
The biochemical endpoint of ferroptosis, i.e., unrestricted/uncontrolled and severe lipid peroxidation, is reached via two major pathways (
Figure 1B)
[9][12]: first, the intrinsic or enzyme-regulated pathway relies on the inhibition of the activity of intracellular antioxidant systems—in the case of ferroptosis, especially the glutathione-dependent GPX4 system (glutathione peroxidase 4); second, the extrinsic or transporter-dependent pathway involves the (de-)regulation of amino acid transporters (system x
c-, which imports cystine as a glutathione (GSH) precursor) or altered iron transport (via transferrin) or both, leading to an increase in the intracellular (labile) iron pool. From an experimental/methodological point of view, an operational definition of ferroptosis
[13] involves the following requirements: cell death (by ferroptosis) is suppressed by both iron depletion (e.g., by ferrostatin 1) and lipophilic radical-trapping antioxidants (liproxstatin-1, vitamin E). Additionally, the direct detection of lipid peroxidation could be considered as further proof of ferroptosis induction
[13].
2. The Translation of Ferroptosis into Clinical HCC Practice
This chapter deals with the issue of how the basic knowledge of (pharmacologically) induced ferroptosis could be translated into “daily” clinical practice for HCC patients. In particular, possible therapeutic scenarios for HCC treatment based on the combination of ferroptosis induction with standard chemotherapy, targeted and immunotherapy as well as local ablative techniques and radiotherapy will be presented on the basis of “pre-clinical” experiments or first clinical trials.
2.1. Ferroptosis Scoring System
The first and more theoretical approach for integrating ferroptosis in the difficult process of optimizing HCC treatment modalities was to develop a predictive and prognostic ferroptosis scoring system (see
Table 1 for an overview). Besides combinatory treatment strategies, the in silico analysis of ferroptosis-related genes in HCC seems to be an interesting approach: Gao et al. developed a scoring model based on these genes for prognosis and immunotherapy response prediction and tumor microenvironment evaluation in HCC samples derived from TCGA (The Cancer Genome Atlas,
n = 377) and GEO databases (Gene Expression Omnibus,
n = 115)
[14]. This research demonstrated that (i) the ferroptosis gene cluster, called Ferrcluster B, and a high ferroscore group is linked to lower overall survival and that (ii) a high ferroscore group classification is associated with the specific in situ expression pattern of programmed death-ligand 1 (PD-1) and to the efficacy of immune checkpoint inhibitors against PD-1 or PD-1 plus cytotoxic T-lymphocyte associated protein 4 (CTLA4).
Table 1. Overview of in silico analysis of ferroptosis genes to develop a predictive and prognostic ferroptosis scoring system for HCC and CCC based on publicly accessible database sets.
| Year |
Database(s) (Dataset) |
Basic Cluster Description |
Predictive and/or Prognostic Aspects of the Ferroptosis Cluster |
Ref |
| 2021 |
TCGA: LIHC
GEO: GSE76427 |
Ferrcluster A: “Olfactory transduction” and “cardiac music contraction”.
Ferrcluster B: “mTOR signaling pathway” and “neurotrophin signaling pathway”.
Ferrcluster C: “adipokine signaling pathway”, “tyrosine metabolism” and “PPAR signaling pathway” |
Ferrcluster B: Overall survival ↓
High ferrscore group: Survival ↓, Programmed cell death 1 (PD-1) mRNA expression ↑, efficacy of PD-1 or PD-1 plus CTLA4 (cytotoxic T-lymphocyte associated protein 4) inhibitors ↓. |
[14] |
| 2021 |
TCGA
ICGC |
Ferroptosis-H and Ferroptosis-L: According to ferroptosis
gene expression and methylation |
Ferroptosis-H: Overall and disease-specific survival ↓ |
[15] |
| 2021 |
GEO
TCGA
ICGC |
C1: Metabolism low, immunity high subtype.
C2: Metabolism high, immunity low subtype. |
C1: Prognosis ↓
C1: Patients with clinical characteristics such as younger, female, advanced stage, higher grade, vascular invasion. |
[16] |
| 2020 |
GEO: GSE14520/GPL3921
TCGA |
Low and high group: Comprehensive index of ferroptosis and immune status (CIFI). |
High CIFI: Prognosis ↓ |
[17] |
| 2021 |
TCGA |
Low-risk and high-risk groups: 2 ferroptosis-related mRNAs and ferroptosis-related lncRNAs |
Higher risk group: Prognosis ↓
Higher risk group: Differences of tumor microenvironment, immune cell infiltration as well as tumor-related pathways |
[18] |
| 2021 a |
TCGA-CHOL
GEO: GSE107943
EMBL-EBI: E-MTAB-6389 |
Low and high group: Ferroptosis-related weighted coexpression gene network and model construction. |
Higher risk group: Prognosis ↓ |
[19] |
a…tumor entity = CCC, all other studies = HCC. Abbreviations: CCC = cholangiocarcinoma, EMBL-EBI = European Molecular Biology Laboratory—European Bioinformatics Institute, GEO = Gene Expression Omnibus, HCC = hepatocellular carcinoma, ICGC = International Cancer Genome Consortium, TCGA = The Cancer Genome Atlas. ↓ means and stands for "less" or "lower" in the relevant context.
Deng et al. could identify two ferroptosis activity-associated subtypes using transcriptome and methylome data from 374 HCC cases with 41 ferroptosis-related genes. Based on these findings, they designed and validated a 15-gene ferroptosis-related prognostic model (FPM) for HCC for accurate risk stratification in a second database with an additional 232 HCC cases from another independent cohort
[15]. Patients with the so called “Ferroptosis-H” phenotype show worse overall and disease-specific survival, which is linked to specific molecular subtypes including mRNA expression patterns, tumor mutation profiles and micro-environmental immune status.
Next, Liu and co-workers extracted and validated two heterogeneous ferroptosis subtypes from 74 ferroptosis related genes in 3933 HCC samples from 32 datasets, whereby the ferroptosis subtype “C1” was related to a lower metabolism and a higher immunity status as well as the opposite status for the ferroptosis subtype “C”
[16].
Additionally, a comprehensive index of ferroptosis and immune status (CIFI) was constructed by combining data from FerrDb and ImmPort with datasets of the GEO GSE14520 (
n = 220) and the TCGA (
n = 365) database
[17]. The authors demonstrated that the subgroup of patients with a high CIFI value had a worse prognosis linked to increased suppressors of ferroptosis paralleled by immunosuppressive cells like cancer-associated fibroblasts (CAFs) and myeloid-derived suppressor cells (MDSCs). The authors concluded and postulated that this CIFI has predictive and prognostic potency for the selection of patients for immunotherapies and targeted therapies.
Zi-An Chen et al. developed a predictive and prognostic ferroptosis-related signature model based on 2 ferroptosis-related mRNAs (SLC1A5 and SLC7A11) and 8 ferroptosis-related lncRNAs (AC245297.3, MYLK-AS1, NRAV, SREBF2- AS1, AL031985.3, ZFPM2-AS1, AC015908.3, MSC-AS1) in HCC
[18]. The findings revealed differences of tumor microenvironment and immune cell infiltration as well as tumor-related pathways between low- and high-risk groups according to the established ferroptosis-related signature model in HCC. Interestingly, the authors could identify 10 significant candidate drugs by integrating in their findings in the L1000FWD database, which could be helpful for further experimental steps for targeting HCC.
In relation to HCC, Zhang et al. constructed a ferroptosis score based on detailed in silico analysis of three different databases with total of 174 cases to predict the efficacy and prognosis of patients with cholangiocarcinoma treated with photodynamic therapy
[19]. Furthermore, the authors could verify and transfer their in-silico-findings by immunohistochemistry, western blot and RNA microarray analyses in vitro and in vivo indicating the reproducibility of such “theoretical” data. As ablative techniques like transarterial embolization (TAE), transarterial chemoembolization (TACE), transarterial radioembolization (TARE), radiofrequency or (RFA) and microwave ablation (MWA) that generate ROS in situ are also routinely applied to HCC, transferring ferroptosis scores could also impact on prediction/prognosis of such ablative techniques in HCC, although clinical validation is still pending
[20].
Finally, the study of Ji Feng et al. could demonstrate that ACSL4, a ferroptosis-promoting enzyme, represents a predictive biomarker for sorafenib sensitivity in HCC in vitro and in vivo
[21]. The investigation of expression of ACSL4 in HCC tumor specimens revealed that the high baseline expression of ACSL4 in untreated HCC tissue is related to complete or partial responses to sorafenib treatment in comparison to the HCC group with low ASCL4 expression
[21].
In summary, the mentioned in silico analyses convincingly indicated that the expression of ferroptosis-associated genes should be integrated as a new predictive and prognostic biomarker in the established classification of HCC
[22]. Patients with HCC could have the benefit of such ferroptosis-related sub-classification with regard to the emerging use of immune checkpoint inhibitors
[20]—provided that such classifiers can be successfully clinically validated.
2.2. Nanoparticles and Exosomes
Next, a more active and more therapeutic approach using nanoparticles or exosomes to integrate ferroptosis into the HCC therapy concept is currently being explored. The basic concept is based on a double carrier model to transfer a ferroptosis inducer in combination with a chemotherapeutical or targeting drug to the cancer cells.
Qiao-Mei Zhou et al. developed iron-doped nanoparticles containing doxorubicin. Doxorubicin and iron act synergistically on the induction of tumor cell death via ferroptosis and apoptosis
[23]. Furthermore, this platform with a superparamagnetic framework could be used to monitor treatment under T2-weighted magnetic resonance imaging as well
[23]. Therefore, the authors concluded that such a nanoplatform could integrate cancer diagnosis, treatment and the monitoring of HCC.
Tang and colleagues designed a dual GSH-exhausting sorafenib loaded manganese-silica nanodrug for inducing ferroptosis in HCC cells via the consumption of intracellular GSH and the inhibition of intracellular GSH synthesis
[24].
Xu et al. constructed a manganese porphyrin-based metallo-organic framework to be used as a nanosensitizer to self-supply oxygen (O
2) and to decrease GSH for ultrasound-triggered sonodynamic therapy. The authors could show strong anticancer and anti-metastatic activity in an in vivo model with hepatocellular and breast carcinoma of the mouse (H22 and 4T1), which was interestingly paralleled by an immunosuppressive microenvironment through increased activated CD8+ T cells and decreased myeloid-derived suppressor cells in situ
[25].
Another nanoparticle-based approach used a cascaded copper-based metallo–organic framework nanocatalyst which bears the cyclooxygenase-2 inhibitor meloxicam and the targeted agent sorafenib to amplify the efficiency of HCC therapy by ferroptosis
[26].
Finally, Ou et al. chose LDL-DHA nanoparticles to induce ferroptotic related cell death in HCC
[27]. Based on their experimental setting, they could convincingly demonstrate that LDL-DHA-treated HCC cell lines in vitro and tumors in vivo exhibited ferroptotic cell death through increased levels of tissue lipid hydroperoxides and the suppression of GPX4 expression
[27].
Another interesting approach was performed by Do et al. who designed exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy
[28]. In detail, the authors developed exosome donor cells (HEK293T) that were transfected with CD47-overexpressing plasmid and loaded with erastin and a photosensitizer (Rose Bengal, RB). These drug-loaded exosomes (Er/RB@ExosCD47) could significantly induce ferroptosis both in vitro and in vivo in tumor cells after laser irradiation at 532 nm without showing toxicity in normal liver
[28].
Highly sophisticated nanoparticle-based platforms delivering ferroptosis-inducing drugs in combination with standard drugs for HCC seem to be a promising step towards integrating ferroptosis into the treatment “portfolio” of HCC in the future.
2.3. Long Noncoding RNAs/miRNA
Another approach is based on the interaction of ferroptosis and either long noncoding RNAs (lncRNAs) or miRNA as identified via in silico
[18][19][29] or via in vitro/in vivo analyses
[30].
Looking in detail at sophisticated in silico techniques, Wang et al. could identify a ferroptosis-specific lncRNA signature, which could serve as an independent prognostic biomarker for the overall survival of patients with HCC. These five extracted lncRNAs (LUCAT1, AC099850.3, AL365203.2, AL031985.3, AC009005.1) could be linked to an HCC specific tumor microenvironment (especially dendritic cells (DCs), macrophages, mast cells, follicular helper T cells, Th1/2 cells, Th2 cells and regulatory T cells) and to the anti-cancer ability of immune checkpoint inhibitors to predict the response to immunotherapy in HCC
[31].
Next, Huang et al. applied various bioinformatics methods to crystallize an immune- and ferroptosis-related lncRNA signature for the prognosis of HCC based on the following 17 candidate LncRNAs after filtration: AC009005.1, AC016773.1, AC090164.2, AC092119.2, AC099850.3, AL021807.1, AL356234.2, AL359510.2, CASC9, DUXAP8, GDNF-AS1, LINC01224, LINC01436, LINC02202, LUCAT1, PTGES2-AS1, and ZFPM2-AS1
[29].
The application of the designed immune- and ferroptosis-related (IF) lncRNAs signature (finally on eight lncRNAs) predicts a worse outcome in patients with HCC that have a high IFlncRNA signature in comparison with those with a low IFlncRNA signature
[29]. Comparable to the in-silico results of Wang et al. the IFlncRNA signature could be correlated to inflammatory cell infiltrates and the expression of immune checkpoints highlighting the potential predictive potency of such an IFlncRNA signature for the response to immune checkpoint inhibitor treatments for HCC. Interestingly, the comparison of the half maximal inhibitory concentration (IC50) of 30 anti-tumor drugs on patients with HCC and an IFlncRNA signature revealed that patients with high IFLSig should show no benefit from gefitinib, mitomycin, temsirolimus and erlotinib on the one hand, but a possible benefit from bexarotene, metformin, sorafenib, bleomycin and lapatinib on the other hand
[29]. Therefore, the researchers suggested that IFLSig could help for the precise selecting chemotherapeutic drug against HCC in relation to the possible clinical benefit.
As mentioned before, Zi-An Chen et al. could develop a Ferroptosis-related signature predictive and prognostic model based on 2 ferroptosis-related mRNAs (SLC1A5 and SLC7A11) and 8 ferroptosis-related lncRNAs (AC245297.3, MYLK-AS1, NRAV, SREBF2- AS1, AL031985.3, ZFPM2-AS1, AC015908.3, MSC-AS1) in HCC
[18].
Integrating the three sets of lncRNA in a classical Venn diagram revealed the most overlap for AC009005.1, AC099850.3 and LUCAT1, which was shown to play a relevant role for autophagy
[32] and could amplify ferroptosis by degradation of ferritin
[33].
When looking at single miRNAs, it could be shown that the miRNA 214-3p (miR-214) plays a regulatory role in the hepatocarcinogenesis via the enhancement of erastin-induced ferroptosis and targeting activating transcription factor 4 (ATF4) in hepatoma cells
[30]. Another study demonstrated that the circular RNA circ0097009 is significantly upregulated in HCC cell lines and tissues and acts as a competing endogenous RNA to regulate the expression of SLC7A11, a key regulator of cancer cell ferroptosis, by sponging miR-1261 in HCC
[34]. Interestingly, expression profiles of genome-wide circRNAs in three pairs of HCC cell lines (before and after sorafenib treatment) revealed that circular RNA hsa_circ_0008367 could positively regulate sorafenib-induced ferroptosis via suppressing ALKBH5-mediated autophagy inhibition
[35]. Finally, Zhang et al. found RNA-binding protein ELAVL1/HuR-dependent ferroptosis in hepatic stellate cells
[36].
More insights on definitive regulative mechanism of non-coding RNAs on ferroptosis in HCC could support and enhance the efficiency of HCC treatment in the coming years.
3. Conclusions
To conclude, ferroptosis seems to harbor potential prognostic and anti-cancer properties in HCC. Despite its dualistic role in the liver, where ferroptosis might be involved in the development of liver pathologies, substances such as RSL-3 or haloperidol can induce ferroptosis, attenuate carcinogenesis and sensitize HCC cells to commonly used therapies. Furthermore, ferroptosis-associated genes show promising features in HCC prognosis and classifying HCC-patients, highlighting a future application in clinical practice. In recent years, ferroptosis has become an interesting and attractive potential approach for cancer treatment in various tumor entities. The concept of ferroptosis was suggested the first time in 2012 by Dixon, characterized by lipid peroxidation and a distinction from apoptosis and necroptosis morphologically, genetically, and mechanistically. Since then, inducing ferroptosis experimentally with FINs, such as RSL-3 or Erastin, shows promising results in targeting cancer cells and could therefore display an alternative type of cell death induction beside the well-known apoptosis.
In cancers that display resistance towards common therapeutic strategies and are highly metastatic, GPX4 and NRF2, two factors influencing ferroptosis negatively, seem to drive cancer resistance
[37]. Therefore, therapy-resistant cancers are more vulnerable to ferroptosis, highlighting a potential role of ferroptosis in drug resistance circumvention
[37].
In HCC, the dismal outcome, caused by acquired resistance towards common therapy, elicits an urge for alternative therapeutic options. So far, current studies display promising anti-HCC activity, as well as the circumvention of sorafenib-resistance.
Although ferroptosis based therapy is promising for cancer in general, current results were only obtained in vitro
[38]. That is because RSL-3 and erastin display specific solubility and metabolic properties, which do not yet allow for in vivo use
[38]. Furthermore, ferroptosis induction in HCC can also damage other healthy cells and tissues due to the unspecificity of FINs
[39]. Moreover, FINs can induce cell death and DNA damage in healthy bone marrow cells, and undesirable side effects in ferroptosis vulnerable organs such as the heart and the kidney are observed
[38][40]. Another important point that needs to be elucidated is the dual role of ferroptosis in HCC. On the one hand, ferroptosis contributes to liver pathogenesis and cancer development and on the other hand, ferroptosis can hamper carcinogenesis, if HCC is established.
One alternative way to circumvent potential side effects of FINs, is the usage of nanoparticles and exosomes that could directly deliver the substances, together with other cancer-therapeutics, to the tumor and induce successfully ferroptosis. Another approach might be to target miRNAs/long non-coding RNAs in HCC, as it has already been demonstrated that miRNAs (miR-214-3p), for instance, can influence ferroptosis positively.
Noncoding RNAs that are associated with ferroptosis might also serve well as potential prognostic biomarkers to predict the overall survival of HCC patients. Furthermore, current evidence suggests, aside from non-coding RNAs, ferroptosis-associated genes and scoring systems in HCC can be used as diagnostic and predictive biomarkers to identify, for instance, specific HCC subgroups to further evaluate eligibility for targeted therapy, especially for immunotherapy. Therefore, ferroptosis in HCC shows not only anti-cancer properties but may also qualify for potential translation in the clinical practice, as diagnostic and predictive biomarkers.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14071826