Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Cardiovascular disease (CVD) is the leading cause of death worldwide. Current data suggest that patients with cardiovascular diseases experience more serious complications with coronavirus disease-19 (COVID-19) than those without CVD. In addition, severe COVID-19 appears to cause acute cardiac injury, as well as long-term adverse remodeling of heart tissue. Cardiac fibroblasts and myofibroblasts may play a pivotal role in both contributing to the deleterious effects of COVID-19-induced cardiac injury, as well as the healing process after cardiac injury.
- TGF-β1
- fibroblast
- myofibroblast
- fibrosis
- COVID-19
1. Introduction
According to the World Health Organization, in 2019, approximately 17.9 million individuals died due to cardiovascular disease (CVD), accounting for 32% of global deaths [1]. The rise of coronavirus disease-19 (COVID-19) is expected to only exacerbate climbing CVD statistics due to cardiovascular injury caused by the infectious disease [2]. Many COVID-19 patients have an increase in cardiovascular damage, particularly acute cardiac injury [3,4], typically indicated by increased troponin levels [5]. In one study performed by Moody et al. [3], 79 hospitalized patients with COVID-19-induced pneumonia underwent two separate transthoracic echocardiographs (TTE), one being a baseline at a median of 8 days after admission and one being 3 months after the baseline. From the baseline TTEs, 55% of patients showed evidence of adverse ventricular remodeling. After 3 months, 29% still had evidence of adverse ventricular remodeling, indicating that cardiovascular damage caused by COVID-19 may be long-term. Moreover, patients with pre-existing CVD are at a higher risk of severe disease than those without [6]. However, due to the novel nature of SARS-CoV-2, its effects on the cardiovascular system are not well understood. This has caused great interest within fields of research and medicine alike to elucidate why SARS-CoV-2 is more fatal to patients with CVD and how it can exacerbate pre-existing cardiovascular damage. The cardiac fibroblast is a well-studied cell type responsible for maintenance and wound healing within the heart. Additionally, cardiac fibroblasts are known for their role in contributing to cardiac fibrosis and adverse remodeling. The fibroblast/myofibroblast’s properties as both healing and harming within the heart make them promising candidates for future research concerning COVID-19-related cardiac injury.
Cardiac fibroblasts are the most prevalent cells in the heart [7]. They are identified using cytoskeletal marker vimentin and cell surface marker discoidin domain receptor 2 (DDR2) [7,8]. Fibroblasts maintain the extracellular matrix (ECM) and the structure of the heart through the production of interstitial collagen [9]. In addition, cardiac fibroblasts are widely known for their response to injury, such as myocardial infarction (MI) [10]. Typically when myocardial infarction occurs, fibroblasts differentiate into an activated phenotype (myofibroblasts) through the activation of transforming growth factor-beta 1 (TGFβ-1) via angiotensin II (Ang II) [9]. Fibroblast activation may also be induced by the release of cytokines, various growth factors, or neurohumoral pathways [11]. Myofibroblasts are a specialized phenotype of cardiac fibroblasts, which express alpha-smooth muscle actin (α-SMA), vimentin, and pro-remodeling factors such as endothelin-1 (ET-1) and Ang-II [9]. This allows for the increased production of collagen and other components beneficial for wound healing. Eventually, scar formation occurs, ideally signaling the apoptosis of myofibroblasts and leaving a healed scar behind [9].
The pathological conditions that can arise when fibroblasts differentiate into myofibroblasts are well-studied. After wound healing, when myofibroblasts fail to undergo programmed cell death, their persistence in the heart may cause excessive collagen synthesis and accumulation in the myocardial interstitium, eventually resulting in adverse remodeling and fibrosis [10,12,13]. Recently, new molecular factors contributing to fibroblast-myofibroblast differentiation and fibrosis have been discovered [5,14]. This includes increased levels of Ang II in COVID-19 patients due to the initial mechanism of binding for the SARS-CoV-2 virus. Increased Ang II levels may accelerate cardiac fibroblast to myofibroblast differentiation [9]. Additionally, microRNAs (miRNAs), specifically miR-125b, seem to be upregulated in fibrotic human hearts and myofibroblasts [14]. However, because of our limited knowledge of the different processes behind fibroblast–myofibroblast differentiation, unknown mechanisms could be contributing to the accumulation of collagen, leading to cardiac dysfunction.
2. Cardiac Fibroblasts in Response to Injury
The cardiac fibroblast comes as a major benefactor to the phases of inflammation, the proliferation of non-myocytes, and scar formation by creating connective tissue in the heart. In response to an injury in the myocardium, inflammation signals activate cardiac fibroblasts, leading them to emit cytokines, prostaglandins, and leukotrienes [15,16], as well as matrix metalloproteinases (MMPs) to clear the injured area of necrotic cells and debris [17]. The produced cytokines induce fibroblast to myofibroblast differentiation, which then furthers the inflammatory response [17].
In a healthy heart, fibroblast activation occurs to provide stronger pro-remodeling factors for the injury. As seen in Figure 1, while resident fibroblasts make up a majority of activated fibroblasts in the heart [9], circulating hematopoietic cells, endothelial cells, progenitor stem cells, and pericytes [10] are speculated to become myofibroblasts as well. It remains unclear if they truly do. However, if these speculated cell types do contribute to the proliferation of activated fibroblasts, they may evoke further cardiac problems [10]. Once activated, more fibroblasts and immune cells are attracted to repair the site of injury in the heart. This could be happening through the cytokines and other mediators that are released by these cells or coming from circulation [9].
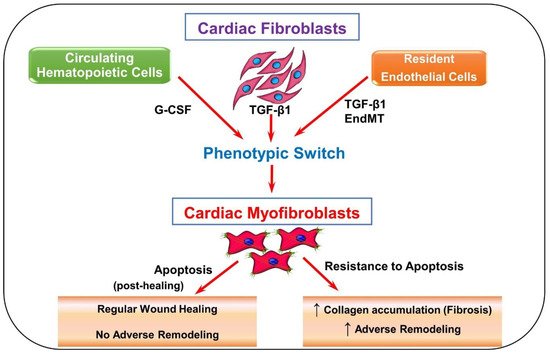
Figure 1. Schematic depicting fibroblast to myofibroblast differentiation. Transforming growth factor-beta 1 (TGF-β1) is the most common pathway involved in the differentiation of fibroblasts into myofibroblasts. Resident fibroblasts, endothelial cells, and circulating hematopoietic cells may have the ability to become myofibroblasts via TGF-β1-induced pathways, granulocyte colony-stimulating factor (G-CSF), and endothelial-to-mesenchymal transition (EndMT). Progenitor stem cells and pericytes are also speculated to contribute to the myofibroblast population. Once myofibroblasts are differentiated, they produce more collagen and various pro remodeling factors required for healing. The expression of these factors at the site of injury promotes repair and remodeling of the injured myocardium. Usually after repair, myofibroblasts undergo apoptosis, decreasing the amount of collagen and pro remodeling factors expressed. When myofibroblasts fail to undergo apoptosis, more collagen and pro remodeling factors are expressed.
Cardiac fibroblast activation unlocks a variety of phenotypic characteristics, such as the enhanced production of collagen facilitated by pro-remodeling factors, including ET-1, Ang II, and TGF-β1 [9]. Myofibroblasts increase collagen turnover to repair damaged heart tissue at the site of injury and in healthy tissue near the injury [10]. As regenerative tissue forms, the collagen’s strength increases as it comes in contact with the injured area [10]. Myofibroblasts also express alpha-smooth muscle actin (α-SMA), a contractile protein that allows the cells to assist in closing wounds [7,9]. The release of these pro-remodeling factors is used to build a stronger ECM and connect the newly created regenerative tissue. Generally, α-SMA is expressed at the site of myocardial injury. Apart from myofibroblasts, α-SMA is also expressed by vascular smooth muscle cells, which are increased in the vessels and surrounding areas after injury [18]. Sometimes this poses a problem in locating myofibroblasts, specifically in areas close to injured vessels.
After the granulation tissue is produced, myofibroblasts typically undergo apoptosis to allow the scar to mature. Failure to undergo apoptosis can result in excessive collagen production [7,9].
Cardiac Fibrosis and Adverse Remodeling
Cardiac fibrosis is the result of excessive accumulation of fibrous ECM proteins in the heart, which progressively leads to cardiac dysfunctions and, ultimately, heart failure [12]. The most robust forms of fibrotic tissue or scar formation occur in response to acute myocardial injury, but other factors such as age, obesity, diabetes, and hypertension are also known to contribute to cardiac fibrosis [12]. When myocardial infarction occurs, a scar must be produced to prevent the rupture of the dead myocardium, as the damaged cardiomyocytes are permanently lost [19]. Although this remodeling is beneficial for repair, excessive changes to the normal ECM structure can have adverse effects [20]. This process initially increases the deposition of ECM proteins causing the loss of contractile function of the heart tissue due to myocardial stiffness and disrupted communication between the cardiomyocytes [7,9,10]. Adverse remodeling is also exacerbated by the loss of cardiomyocytes through necrosis and apoptosis [13]. As the fibrosis continues to progress, left ventricle hypertrophy and systolic dysfunction may occur, potentially giving way to heart failure [7,9]. Altogether, these factors encompass a compilation of harmful effects on cardiac structure and function at various magnitudes [13].
The most well-studied fibroblast activation pathway begins when Ang II activates TGF-β1 through the TGF-β1 signaling pathway, inducing a phenotypic switch. This signaling continues to take place, leading to the accumulation of myofibroblasts in the myocardium. TGF-β binds to the TGF-β-receptor II, which phosphorylates TGF-β receptor kinase 1 (TβR1) and activates SMAD-dependent TGF-β canonical pathway. This, in response, induces fibrosis [10]. To stop the accumulation of myofibroblasts in the heart, scientists suggest that certain signaling pathways, specifically TAK1 and p38, should be inhibited [10].
According to Ono et al. [21], TGF-β-activated kinase 1(TAK1) increases ECM protein production, contributing to the development of fibrotic disorders. To stop this, a dominant-negative TAK1 inhibitor (TAK1-DN) needs to bind with the kinase. From their experiment, TAK1-DN proved to reduce ECM protein production and has the ability to intercept TGF-β1 signaling. Additionally, in a study by Li et al. [22], when upregulating activating transcription factor-3 (ATF3) expression, MAP2K3 expression was suppressed and later inhibited p38 signaling. Inhibition of p38 reduced the expression of TGF-β signaling-related genes. ATF3 is notably a responsive gene in cardiac fibroblast activation [23]. When activated, the gene proceeds to regulate the cellular mechanisms with other ATF genes by activating or repressing working genes [23]. In this case [22], ATF3 inhibits p38 signaling making its upregulation during cardiac failure a self-protective mechanism from fibrosis and, consequently, adverse remodeling. Thus, inhibiting cell signaling pathways that induce the incident of fibrosis could come as a potential therapeutic approach for individuals with MI caused by cardiac fibrosis. Furthermore, manipulating signaling pathways has come as an insightful way to determine what mechanisms are present for cardiac fibrosis to occur.
3. Emerging Cardiac Conditions Relating to COVID-19
Recent literature indicates that CVD is a risk factor for severe COVID-19 and is defined as a cardiac tissue insult in response to the virus’ interaction. Further myocardial damage is likely to be exhibited if a SARS-CoV-2 patient initially had a CVD [24]. Among the 44,672 cases of COVID-19 reported by the Chinese Center for Disease Control and Prevention, the population determined a case-fatality rate of 10.5% with pre-existing comorbid CVDs [25]. Furthermore, a meta-analysis performed by Li et al. [26] identified that among the 1527 patients with COVID-19 in Wuhan, China, the prevalence of hypertension and cardiac/cerebrovascular disease was 16.7%, 17.1%, and 16.4%, respectively. Acute myocardial injury was reported in at least 8% of these patients and tended to have a higher incidence rate in ICU COVID-19 patients [26].
3.1. COVID-19-Induced Cytokine Storm
The infiltration of SARS-CoV-2 into respiratory epithelial cells activates an immune response characterized by the production of pro-inflammatory cytokines [27]. This includes interleukin 6 (IL-6), IL-12, IL-1β, and interferon γ [28]. The overproduction of these cytokines and chemokines causes a “cytokine storm”, is often an indicator of severe disease, and precipitates acute respiratory distress syndrome [29]. In a meta-analysis by Coomes and Haghbayan [30], including 10 studies with cytokine levels reported in COVID-19 patients, all studies found increased levels of IL-6, with five studies finding higher levels in patients with severe disease compared to those with moderate disease.
The expression of cytokines, particularly IL-1β and IL-6, is also prominent in fibrotic hearts [12]. Hypertension is a known risk factor for fibrosis, and increased plasma IL-6 levels, mediated by Ang II, tend to correspond with increased systolic blood pressure and decreased endothelial function [31], indicating that IL-6 may play a role in increasing blood pressure and decreasing cardiac function [32]. Additionally, cytokine release can induce cardiac fibroblast activation, potentially promoting fibrosis and adverse remodeling [11]. Thus, the overproduction of said cytokines and chemokines is likely contributing to the cardiac damage seen in severe COVID-19 patients.
3.2. ACE2’s Involvement in Viral Infection
Initially, SARS-CoV-2 binds to ACE2 receptors notably expressed in the lungs, heart, and vessels but found throughout the body as well. In a study published by Zou et al. [33], the proportion of cells expressing ACE2 in several tissues throughout the body was calculated. It was determined that tissues with >1% ACE2 expression were at high risk for viral infection. The tissues which fit this distinction are as follows: ileum (30%), heart (>7.5%), kidney (4%), bladder (2.4%), respiratory tract (2%), and esophagus (>1%) [33]. The high proportion of ACE2 expressing cells in the heart may explain why severe myocardial injury is often a symptom of severe COVID-19.
When SARS-CoV-2 interacts with ACE2, the enzyme loses contact with the cell surface, causing Ang II levels to increase and Ang-(1–7) levels to decrease, as seen in Figure 2 [5,34]. Recent studies have demonstrated elevated levels of autoantibodies targeting angiotensin II type 1 receptor (AT1R) in patients with moderate to severe COVID-19 compared to milder cases [35] and versus healthy controls [36]. This indicates an immune response similar to that of autoimmune disease. Activation of AT1R, typically via elevated Ang II expression, can thus induce cardiac fibroblast differentiation activity and ultimately lead to the proliferation of myofibroblasts [9]. Hence, this mechanism may explain why patients with COVID-19 and underlying cardiac conditions show vascular inflammation, endothelial dysfunction, and thrombosis. Since ACE2 is widespread in the body, increased Ang II levels may also occur in organs other than the heart and create inflammation in more advanced COVID-19 patients [5]. In addition, the renin–angiotensin–aldosterone system (RAAS) is affected by sex, as is the expression of ACE2. In male cardiac cells, levels of soluble angiotensin-converting enzyme 2 (sACE2) tend to be increased [37]. It has been theorized that increased levels of ACE2 could explain why males tend to have worse COVID-19 outcomes and increased mortality compared to females [38].
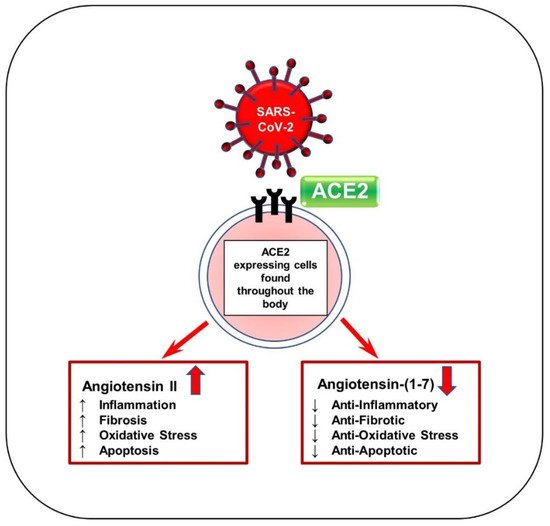
Figure 2. Schematic of SARS-CoV-2 viral entry via ACE2 receptor. SARS-CoV-2 enters the body by first binding to ACE2 receptors by the S protein. ACE2 is responsible for generating Ang-(1–7) from Ang II and acts mainly to reduce blood pressure. This binding can block the ACE2 receptor and reduce ACE2 activity, thereby significantly increasing Ang II levels and drastically decreasing Ang-(1–7) levels. Ang-(1–7) serves as anti-inflammatory, antifibrotic, antioxidative, and antiapoptotic. Ang II increases hypertension and other associated factors such as inflammation, fibrosis, oxidation, and apoptosis.
Over the past year, it has been noted that the mortality rate for individuals with underlying chronic diseases, specifically cardiometabolic diseases, has surged compared to the time before the pandemic [39]. A wide range of cardiac complications is present in patients with COVID-19 that have significantly worsened their health, including myocardial injury, myocarditis, acute myocardial injury, and heart failure [40]. Myocardial infiltration of immune cells, activation of myofibroblasts, and cardiomyocyte necrosis are a few of the short-term complications of COVID-19 that could lead to inflammation; local and systemic inflammation plays a significant role in further evoking existing cardiac conditions. Long-term effects that could be caused by inflammation include cardiac fibrosis, cardiac hypertrophy, and decreased cardiac output [41], as well as ischemic myocardial injury with its other related mechanisms such as plaque disruption, coronary spasm, and microthrombi. These conditions could eventually lead to cardiac dysfunction, observed as responses to inflammation and, possibly, direct viral action [5].
This entry is adapted from the peer-reviewed paper 10.3390/cells11081316
This entry is offline, you can click here to edit this entry!
