There are different types of face masks (e.g., 3-ply surgical masks, a wide range of fabric masks, single-use face masks, and face shields) and respirators available commercially to protect individuals from getting and/or spreading COVID-19. Face masks are designed to protect primarily from respiratory droplets and particles to some extent. In contrast, the respirators are designed with extended protection against respiratory droplets, particles, and the virus that cause COVID-19. Both face masks and respirators have certain requirements to ensure performance and efficacy at a reliable and consistent level. Mask standards, ratings. National Institute for Occupational Safety and Health (NIOSH), ASTM International, and International Organization for Standardization (formerly known as International Association for Testing Materials) are engaged in creating an open and transparent consensus standards development process for addressing the standards gap identified during the wake of COVID-19 pandemic. The development of standards aimed to define performance requirements for source control, the protective efficacy of face mask or barrier face coverings, and standardized products to inform user selection decisions. The primary purpose of these specifications is to enable source control to protect the mass public with performance requirements such as protection capability, comfort, reusability, and so on. There are a few basic requirements of face masks to be used effectively, including an excellent fit over the nose and mouth of the user to prevent leaking (use of fitter, base, or nose wire) and the use of multi-layer non-woven but breathable fabrics for preventing bright light.
1. Medical Face Masks
Medical face masks, often referred to as disposable face masks or surgical masks, are classified according to three levels of protection: level 1 (low), level 2 (moderate), and level 3 (high), with differing specifications (
Table 1). Fit testing is not required for these masks, typically loosely fitted. They are frequently used in the healthcare setting with a low to moderate risk of acquiring airborne transmissible diseases
[1]. Since there are regulatory restrictions, not all face masks qualify as surgical masks in the United States. The certification of the ASTM standards authority is mandatory for a mask to be qualified as a surgical mask. In Europe, European Standards Organization (EN) certifies surgical masks. Medical face masks must meet a few specifications for filtering according to the ASTM F2100-19e1
[2], including bacterial filtration efficiency (BFE), submicron particulate filtration efficiency (PFE), differential pressure (ΔP, an indicator of the breathability of the mask), flammability, and so on. Both BFE and PFE must meet the requirements of ≥95% for low-barrier face masks (level 1) and ≥98% for moderate- and high-barrier face masks (levels 2 and 3). Surgical N95 respirators and other head/facial PPE (personal protective equipment) have flammability class 1, indicating relatively poor flammability. Compared to other surgical masks, level 3 surgical masks offer the highest filtration efficacy, capable of filtering over 98% of particles of 3.0 μm in size, and have the highest possible fluid resistance
[3]. On the contrary, in the European system, surgical masks are graded into type I-III, having the filtration capability same as their US counterparts. Surgical masks do not filter small particles effectively, although they can protect from directly spattering droplets. As surgical masks are loosely fitted around the face, they cannot prevent leaking from the lateral aspects of the masks
[4]. Consequently, this mask is not recommended by the NIOSH, USA, to be used as particulate. However, surgical masks are capable of effectively blocking large respiratory droplets, splashes, and sprays during routine surgical or medical procedures.
Table 1. Comparison of ASTM F2100-19 standard specification for performance of materials used in medical face masks (USA) and EN 14683:2019 medical face mask requirements and test methods–(EU).
| |
|
ASTM F2100-19 |
EN 14683:2019 Barrier levels |
| Level 1 |
Level 2 |
Level 3 |
Type I |
Type II |
Type IIR |
Barrier
testing |
BFE (%)
ASTM F2101, EN 14683 |
≥95 |
≥98 |
≥95 |
≥98 |
PFE (%)
ASTM F2299 |
≥95 |
≥98 |
Not required |
Splash resistance,
Synthetic blood ASTM
F1862, ISO22609 |
Pass at
80 mmHg |
Pass at
120 mmHg |
Pass at
160 mmHg |
Not required |
Pass at ≥ 16.0
kPa (120 mmHg) |
Physical
testing |
Differential pressure
EN 14683 |
5 mmH2O/cm2 |
6 mmH2O/cm2 |
40 Pa/cm2 |
60 Pa/cm2 |
Safety
testing |
Flammability
16 CFR Part 1610 |
Class 1 (≥3.5 seconds) |
See European Medical Directive
(2007/47/EC, MDD 93/42/EEC) |
Microbial cleanliness
ISO 11737-1 |
Not required |
≤30 cfu/g |
Biocompatibility
ISO 10993 |
510 k Guidance recommends
testing to ISO 10993 |
Complete an evaluation
according to ISO 10993 |
Sampling
ANSI/ASQC Z1.4
ISO 2859-1 |
▪ AQL 4% for BFE, PFE, delta P
▪ 32 masks for synthetic blood
(Pass ≥29 passing, Fail ≤28 passing)
▪ 14 masks for flammability |
▪ Minimum of 5 masks up to an AQL of 4% for BFE, delta P and microbial cleanliness
▪ 32 masks for synthetic blood splash resistance
(Pass ≥ 29 passing, Fail ≤ 28 passing) |
2. Respirator Masks (N95 and FFP2 Variants)
The respirators (
Figure 1) are designed, tested, and evaluated by NIOSH against a specific US standard, including quality requirements. The KN95 respirators are the most widely available and used respirators globally that meet standards at the international level. There are several other respirators of such standards; for example, DL2, DL3, DS2, DS3, FFP2, FFP3, KN100, KP95, KP100, P2, P3, PFF2, PFF3, and R95. An Emergency Temporary Standard (ETS) has been published by the Occupational Safety and Health Administration (OSHA) as the domestic demand and supply for respirators are increasing. As the Centers for Disease Control and Prevention (CDC) and NIOSH are committed to protecting health care workers as the foremost priority, the Food and Drug Administration (FDA) ensures that the health care provider should no longer use crisis capacity strategies. For such reasons, ETS was a necessary and timely step to address the COVID-19 crisis
[5]. However, most commercially available KN95 respirators in different countries are counterfeit and do not comply with the NIOSH standard. For example, around 60% of KN95 respirators available commercially in the USA are fake. Therefore, CDC has published guidelines and standards to choose the right one for specific purposes.
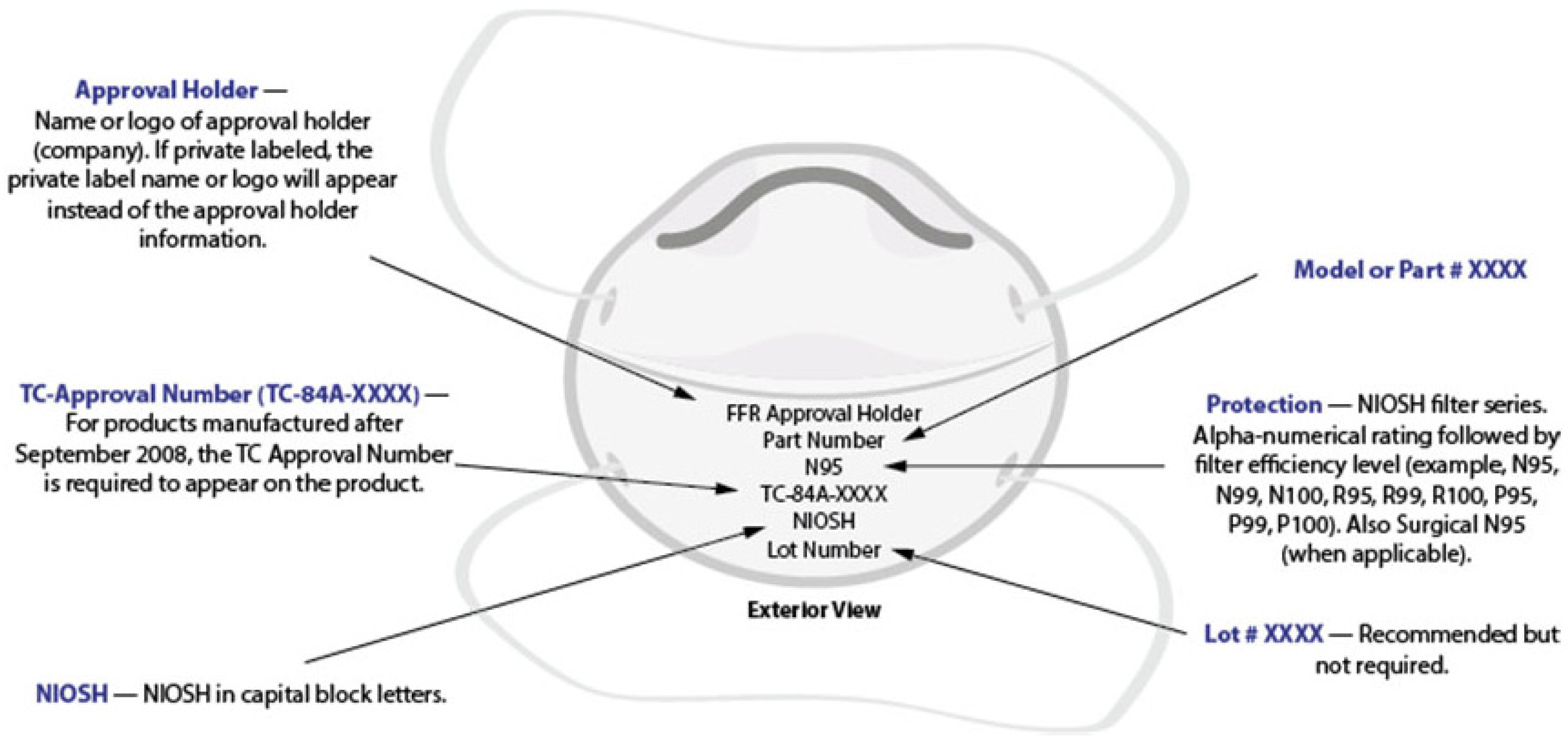
Figure 1. Model of a generic filtering respirator with appropriate markings
[6].
The respirators, class II medical devices regulated by the FDA, should be designed to ensure proper facial fit (i.e., designed to seal around the nose and mouth) and efficiently filter (up to 95%) airborne particles. The layering of the respirator may range between 3–5 layers of fabric and must pass the GB2626-2006 standard. Layer 1 is usually spun-bound made of non-woven cotton, which forms the shape and provides structural supports to the mask. The material should be excellent compatible with human skin. Layers 2 and 3 comprised of 2 filter layers (melt-blown fabric with a fiber diameter of 2 μm) should be capable of filtering dust, bacteria, and pollen. Spun-bound (non-woven cotton) or ES (ethylene-propylene side by side) hot hair cotton, a 2-component composite fiber, makes the layer 4. Layer 5 is made to give structural support and shape, and offers excellent compatibility and non-irritation to the skin of the user. However, the efficacy of the respirator depends on the weight of the material rather than the number of layers. Layers of melt-blown fabric somewhat provide a balance between filtering and breath resistivity. N95 (NIOSH-42CFR84 standard, United States), KN95 (GB2626-2006 standard, China), and FFP2 (EN 149-2001 standard, Europe) are not exactly similar, but they are certified as meeting the standards and can be expected to function very similarly to each other
[7]. However, these masks are made for single use only and recommended to change after every patient encounter. After prolonged use, due to the tight-fitting and high filtering capacity, the wearers of respirator masks may experience discomfort and shortness of breath.
3. Single-Use Face Masks
Single-use face masks (see the image in Table 1) lack the requirements of surgical masks. Although their fabrication varies, single-use face masks are generally thin and made of only a single layer. If the surgical masks are abundant in supply, single-use face masks are not generally recommended to reuse in the healthcare setting. Although single-use face masks usually fail to filter tiny particles, they may still have the reasonably well capability to block the emission of large droplets and saliva. Additionally, in case of scarcity of the supply of surgical masks and respirator masks, single-use face masks can be an alternative to be used in the community setting, even for healthcare workers.
4. Cloth Face Mask
Cloth face masks (
Figure 2a) made from various fabrics are widely available and are being used on a regular basis globally. However, NIOSH has recommended and evaluated such face masks according to the ASTM F3502-21 (standard for barrier face covering). NIOSH recommends wearing a face mask that meets ASTM F3502, ’workplace performance’, and ’workplace performance plus’. ASTM International also published F3050-17: Standard Guide for Conformity Assessment of Personal Protective Clothing and Equipment. The face masks should be able to filter at least 1 in every 5 nano-meter-sized particles from passing through the mask material and reaching the lungs of the user, which means a minimum sub-micron filtration score of 20% to qualify for the ASTM F3502 standard. It is also recommended that the user should either wear two masks combining a cloth mask and disposable mask or a 3-ply mask for better protection from COVID-19
[8].

Figure 2. Various types of face masks: (a) cloth mask, (b) temporary fabric mask, (c) MNP mask or surgical mask, (d) FFP2 mask or N95 mask, and (e) FFP3 mask or respirator.
Because of the severe scarcity of N95 respirators and surgical masks, several countries limit their usage by the general population, reserving them for individuals at the greatest risk of virus infection, such as healthcare professionals who come into contact with sick patients. The CDC, USA, released a recommendation in April 2020 encouraging the use of cloth face masks in public places to reduce the spread of COVID-19. This mask can be a reasonable solution when appropriate masks are in limited supply. Although cloth masks cannot protect against aerosols, they may limit viral transmission if other guidelines are followed, such as remaining at home, restricting unnecessary travel, and maintaining social distance.
The following kinds of masks are available in the context of SARS-CoV-2:
(1) Masks for daily usage (temporary fabric masks, Figure 2b): These masks do not protect against infection to the wearer. However, there is a slight risk decrease for droplet transmission, particularly during exhale, which minimizes the possibility of viral spreading. Although these masks are widely suggested for the general public while strolling, shopping, or using public transport, these masks should not be worn in the medical setting.
(2) MNP (medical mouth–nose protection; see Figure 2c): It is also known as a "surgical mask." For preventing infection, strict restrictions govern the industrial manufacture of MNP. The filtering performance is comparable to that of daily use masks, and they are designed to protect patients. They have been authorized for use by medical personnel, and their main purpose is to protect patients from aerosols.
(3) FFP2 mask (face filtering component, Figure 2d)/N95-mask: FFP2 masks meet stricter safety standards. They shield the wearer’s lungs by preventing more than 95% of particles and droplets from entering the lungs during breathing. As long as there is no exhaling valve, FFP2 masks efficiently protect the environment. In contrast, masks with an exhaling valve enable exhaled air to escape unfiltered, contaminating the environment.
(4) FFP3 mask: FFP3 masks (Figure 2e) protect the user even better than FFP2 because they filter out more than 99% of droplets and particles when inhaled. In the absence of an exhaling valve, FFP3-masks also protect the environment.
This entry is adapted from the peer-reviewed paper 10.3390/polym14071296



