Global environmental pollution has led to human exposure to ultraviolet (UV) radiation due to the damaged ozone layer, thereby increasing the incidence and death rate of skin cancer including both melanoma and non-melanoma. Overexpression and activation of V-akt murine thymoma viral oncogene homolog (AKT, also known as protein kinase B) and related signaling pathways are major factors contributing to many cancers including lung cancer, esophageal squamous cell carcinoma and skin cancer. Although BRAF inhibitors are used to treat melanoma, further options are needed due to treatment resistance and poor efficacy. Depletion of AKT expression and activation, and related signaling cascades by its inhibitors, decreases the growth of skin cancer and metastasis. Here we have focused the effects of AKT and related signaling (PI3K/AKT/mTOR) pathways by regulators derived from plants and suggest the need for efficient treatment in skin cancer therapy.
- AKT
- skin cancer
- AKT inhibitor
- signal transduction
1. Introduction
Skin cancer is one of the most frequent cancers worldwide [1][2], with increasing annual costs and morbidity [3][4]. Based on their cellular origin, skin cancers are mainly divided into melanoma (derived from melanocytes) and non-melanoma skin cancer (NMSC, from epithelial cells). NMSC includes basal cell carcinoma (~80%) and squamous cell carcinoma (~16%) and melanoma accounts for ~4% of all skin cancer [5]. Even though it accounts for a small portion of skin cancer, melanoma is the most malignant cause of invasive metastasis with a five year survival rate of ~20% [6]. An estimated 7,700,000 new cases of NMSC, including 5,900,000 basal cell carcinomas and 1,800,000 squamous cell carcinomas, have been reported globally including 195 countries accounting for 65,000 cancer deaths [2][7]. An estimated ~100,350 new cases of melanoma and 6850 related deaths have been reported in the USA during 2020 [1].
The major environmental risk factors for skin cancer (melanoma and NMSC) include UVA, UVB and UVC [6][8]. UVC has the shortest wavelengths spectrum (100–280 nm) and after absorption by the ozone layer and atmosphere, it barely reaches the earth. Generally, the human skin is affected by solar UV (100–400 nm) radiation composed of UVA (315–400 nm, ~95%) and UVB (280–315 nm, ~5%) rays triggering skin inflammation, carcinogenesis and cancer development [9][10].
Genetic mutation of p53, BRAF, RAS, CDKN2A and PTEN, and abnormal expression/activation of T-LAK cell-originated protein kinase (TOPK), mitogen-activated protein kinase kinase (MEK), 90 kDa ribosomal S6 kinase (RSK) and AKT in melanoma and NMSC induce cancer cell signal transduction thereby promoting skin carcinogenesis and cancer cell proliferation, migration and invasion [6][9][11][12][13][14]. Solar UV triggers PTEN mutations. Other environmental stimuli induce overexpression and/or activation of AKT and related signaling pathways in skin cancer proliferation and survival. Therefore, it is not surprising that AKT-mediated signaling is one of the main mechanisms in skin cancer therapy. The focus of the article is on AKT and related signaling pathways, and their implications in skin cancer therapy.
Strategies for the skin cancer management include surgery, radiation, chemotherapy and cutting-edge targeted therapies [13][15]. Dacarbazine, temozolomide, paclitaxel, cisplatin and carboplatin are generally used to treat melanoma, whereas vismodegib, sonidegib and cetuximab are used to target basal and squamous cell carcinoma. Vemurafenib is a competitive inhibitor of BRAF kinase activity, and especially inhibits melanoma with a V600 mutation. However, treatment with these therapeutic agents is associated with severe side effects and a low quality of life including nausea, hair loss and increased risk of infection. It also increases the risk of resistance immediately after treatment for several months. Therefore, natural compounds are the preferred choice due to their reduced risk of toxicity and cost-effectiveness.
2. AKT and Related Signaling Pathways Are Important in Skin Cancer Regulation
AKT is a serine/threonine-specific protein kinase and alternatively named in protein kinase B (PKB). It plays a key role in multiple cellular processes such as protein synthesis, cell proliferation, cell cycle progression, survival and migration as well as metabolism (Figure 1) [16][17]. The AKT subtypes include AKT1, AKT2 and AKT3 which contain conserved domains such as pleckstrin homology (PH) domain for protein–protein interaction or interaction with phosphatidylinositol phosphate (PIP)-containing lipid bilayers, linker, kinase domain for catalytic activity and regulatory (hydrophobic motif, HM) domain for compete activation with high homology (~80%) as shown in Figure 2. Most of the direct AKT inhibitors target the ATP binding pocket in the kinase and HM domain. In the AKT knock-out (KO) mouse model, each AKT isoform showed different phenotypes such as growth retardation and altered placental development phenotype with increasing lethality in AKT1 KO, insulin resistance and mild growth retardation in AKT2 KO mice, and abnormal development of skin and bone/muscle defect in AKT3 KO [18][19]. AKT is highly activated and expressed in cancers including lung, ovarian, pancreatic and esophageal squamous cell carcinoma [16][20]. In skin cancer, the active form of phospho-AKT (pAKT) was overexpressed in 22 (54%) of 41 benign nevi and 112 (71.3%) of 157 primary melanoma tumors compared to normal adjacent tissue, resulting in a reciprocal five-year survival rate in human metastatic melanoma [21][22]. Together with downstream target protein mTOR, the activated AKT1 induces highly metastatic melanomas involving lung (67%) and brain (17%) in BRAFV600E/Cdkn2aNull mice [23]. Thus, AKT is implicated in melanoma metastasis. AKT protein is activated by the phosphorylation of PI3K (p110/p85 heterodimer), which recruits AKT to the cell membrane through phosphatidylinositol (3,4,5)-trisphosphate (PIP3)-PH domain binding. It’s also stimulated by receptor tyrosine kinase (RTK), phosphatidylinositol-dependent kinase (PDK) 1 or solar UV-induced signaling. The active AKT (p-AKT) mediates the signal transduction to downstream molecules such as the mTOR complex (mTORC)/p70S6 kinase (S6K), caspases, BCL2-associated agonist of cell death (BAD), mouse double minute 2 homolog (MDM2, E3 ubiquitin-protein ligase)/p53, forkhead box protein O1 (FOXO1)/B-cell lymphoma 2 (Bcl-2)/Bcl-2-like protein 11 (Bim)/Bcl-2-associated X protein (Bax), WEE1(G2 Checkpoint Kinase), p27 and glycogen synthase kinase 3 beta (GSK3β/β-catenin/glutamine synthetase (GS)/cyclin D1 for protein synthesis, cell survival, cell cycle, metabolism, migration and invasion (Figure 1). The other hands, PTEN/PI3K/AKT-mediated pathways promote cancer cell migration and invasion by the upregulation of matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor (VEGF) [24][25]. Therefore, it is desirable to regulate the AKT kinase expression and activation and related signaling pathways for skin cancer therapy.
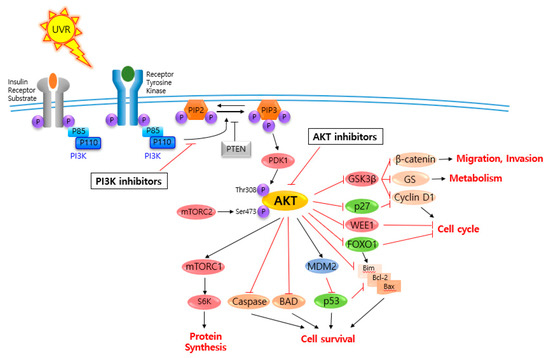
Figure 1. V-akt murine thymoma viral oncogene homolog (AKT) and related signaling pathways are associated with several features of skin cancer.
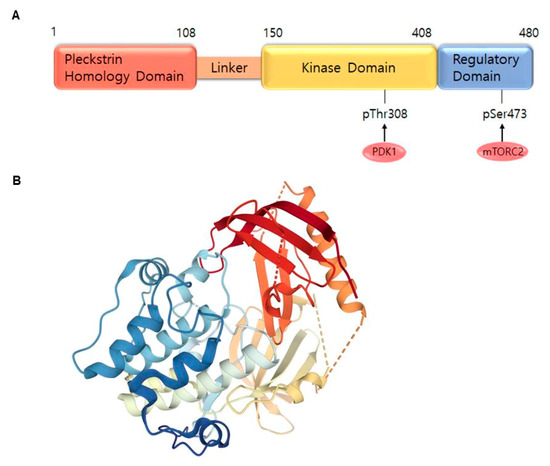
Figure 2. AKT1 structure. (A) Domains of the AKT1. AKT consists of an N-terminal pleckstrin homology (PH) domain (residues 5–108), linker, a catalytic kinase domain (residues 150–408) and a hydrophobic C-terminal tail (HM) (residues 409–480). (B) Crystal structure of AKT1 (PDB ID:3O96). Red indicates N-terminal domains consisting of PH; blue represents HM (http://www.rcsb.org/) (accessed on 18 August 2020).
This entry is adapted from the peer-reviewed paper 10.3390/ijms21186869
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J Clin 2020, 70, 7-30.
- Global Burden of Disease Cancer, C.; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I., et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019, 10.1001/jamaoncol.2019.2996.
- Tran, D.A.; Coronado, A.C.; Sarker, S.; Alvi, R. Estimating the health care costs of non-melanoma skin cancer in Saskatchewan using physician billing data. Curr Oncol 2019, 26, 114-118,.
- Guy, G.P., Jr.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med 2015, 48, 183-187.
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsague, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol Ther (Heidelb) 2017, 7, 5-19.
- Wrobel, S.; Przybylo, M.; Stepien, E. The Clinical Trial Landscape for Melanoma Therapies. J Clin Med 2019, 8.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68, 394-424.
- Khavari, P.A. Modelling cancer in human skin tissue. Nat Rev Cancer 2006, 6, 270-280.
- Roh, E.; Lee, M.H.; Zykova, T.A.; Zhu, F.; Nadas, J.; Kim, H.G.; Bae, K.B.; Li, Y.; Cho, Y.Y.; Curiel-Lewandrowski, C., et al. Targeting PRPK and TOPK for skin cancer prevention and therapy. Oncogene 2018, 37, 5633-5647.
- Lee, M.H.; Lim, D.Y.; Kim, M.O.; Lee, S.Y.; Shin, S.H.; Kim, J.Y.; Kim, S.H.; Kim, D.J.; Jung, S.K.; Yao, K., et al. Genetic ablation of caspase-7 promotes solar-simulated light-induced mouse skin carcinogenesis: the involvement of keratin-17. Carcinogenesis 2015, 36, 1372-1380.
- Kim, J.E.; Lee, K.W. Molecular Targets of Phytochemicals for Skin Inflammation. Curr Pharm Des 2018, 24, 1533-1550.
- Zhao, R.; Choi, B.Y.; Lee, M.H.; Bode, A.M.; Dong, Z. Implications of Genetic and Epigenetic Alterations of CDKN2A (p16(INK4a)) in Cancer. EBioMedicine 2016, 8, 30-39.
- Pal, H.C.; Hunt, K.M.; Diamond, A.; Elmets, C.A.; Afaq, F. Phytochemicals for the Management of Melanoma. Mini Rev Med Chem 2016, 16, 953-979.
- Lee, M.H.; Huang, Z.; Kim, D.J.; Kim, S.H.; Kim, M.O.; Lee, S.Y.; Xie, H.; Park, S.J.; Kim, J.Y.; Kundu, J.K., et al. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev Res (Phila) 2013, 6, 455-465.
- Nikas, I.P.; Paschou, S.A.; Ryu, H.S. The Role of Nicotinamide in Cancer Chemoprevention and Therapy. Biomolecules 2020, 10.
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a Therapeutic Target for Cancer. Cancer Res 2019, 79, 1019-1031.
- Shariati, M.; Meric-Bernstam, F. Targeting AKT for cancer therapy. Expert Opin Investig Drugs 2019, 28, 977-988.
- Kumar, C.C.; Madison, V. AKT crystal structure and AKT-specific inhibitors. Oncogene 2005, 24, 7493-7501.
- Fayard, E.; Tintignac, L.A.; Baudry, A.; Hemmings, B.A. Protein kinase B/Akt at a glance. J Cell Sci 2005, 118, 5675-5678.
- Song, M.; Liu, X.; Liu, K.; Zhao, R.; Huang, H.; Shi, Y.; Zhang, M.; Zhou, S.; Xie, H.; Chen, H., et al. Targeting AKT with Oridonin Inhibits Growth of Esophageal Squamous Cell Carcinoma In Vitro and Patient-Derived Xenografts In Vivo. Mol Cancer Ther 2018, 17, 1540-1553.
- Soares, C.D.; Borges, C.F.; Sena-Filho, M.; Almeida, O.P.; Stelini, R.F.; Cintra, M.L.; Graner, E.; Zecchin, K.G.; Jorge, J. Prognostic significance of cyclooxygenase 2 and phosphorylated Akt1 overexpression in primary nonmetastatic and metastatic cutaneous melanomas. Melanoma Res 2017, 27, 448-456.
- Slipicevic, A.; Holm, R.; Nguyen, M.T.; Bohler, P.J.; Davidson, B.; Florenes, V.A. Expression of activated Akt and PTEN in malignant melanomas: relationship with clinical outcome. Am J Clin Pathol 2005, 124, 528-536.
- Cho, J.H.; Robinson, J.P.; Arave, R.A.; Burnett, W.J.; Kircher, D.A.; Chen, G.; Davies, M.A.; Grossmann, A.H.; VanBrocklin, M.W.; McMahon, M., et al. AKT1 Activation Promotes Development of Melanoma Metastases. Cell Rep 2015, 13, 898-905.
- Li, X.; Yang, Z.; Song, W.; Zhou, L.; Li, Q.; Tao, K.; Zhou, J.; Wang, X.; Zheng, Z.; You, N., et al. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int J Oncol 2013, 43, 793-802.
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002, 2, 161-174.
