Applying innovative diagnostic and therapy techniques in connection with the immune system and viral pathology is essential to meet the greatest medical challenges today, and these topics will be discussed in this entry.
Complex interventions may improve the activity of the immune system and save the lives of people with immune imbalances, such as children suffering from sJIA.
The first goal of this entry, in the COVID era, is to deepen the comprehension of the pathophysiological mechanisms of sJIA and to highlight the best diagnostic methods, including MAS, a life-threatening condition. The second purpose is to correctly identify all symptoms and signs of MAS in sJIA, and hyperinflammation in COVID-19, to rule out the mimickers. The last aim, but not the least, is to raise awareness among researchers, general practitioners and pediatric rheumatologists about current treatment choices and to understand the targeted approach to sJIA-MAS therapy in this developing pandemic, to provide patients with the best care to keep their immune system as balanced as possible.
2. MAS in sJIA and Hyperinflammation in COVID-19
From the published data so far, which is related to MAS and the cytokine storm from a viral infection with SARS-CoV-2 and other viral infections, for example, H1N1, there are multiple common aspects of biochemical and immunological abnormalities. Biological parameters show disorders in blood clotting with increased D-dimers and serum ferritin, high von Willebrand factor, intravascular coagulation with microthrombus formation, and increased vascular endothelial permeability
[34][35][36].
MAS, a severe complication of sJIA, seems to overlap with CSS and has appeared since 2020 in a certain category of patients with COVID-19. The term CSS brings together clinical and biological manifestations at the same time as hyperinflammation, which can compromise the hemodynamic activity and functions of multiple organs, with disastrous consequences for the patient. In several published studies, the authors consider MAS as a prototypical form of CSS that appears as a severe complication in the evolution of several autoinflammatory and/or autoimmune diseases, such as sJIA, AOSD, SLE and KD
[37][38][39].
Analyzing MAS in patients with sJIA, it was found that from a clinical-biological point of view, these data are accompanied by an exaggerated hyperferritinemia, coexisting with hemocytopenia, and coagulopathy of consumption with significant hepatic dysfunction. The predictive prototype for MAS in the febrile patient with sJIA is hyperferritinemia (>684 ng/mL), which will be associated with any two of the following additional criteria: platelet count ≥181 × 10
−9/L, aspartate aminotransferase >48 units/L, triglycerides >156 mg/dL and fibrinogen ≥360 mg/dL. Since hyperinflammation in sJIA is of particular importance and the practitioner must assess in a timely manner whether it is intensely active sJIA or whether MAS is already occurring, differentiation guidelines have been implemented to support the clinician
[40][41][42].
The current prototype supporting MAS pathogenesis is based on the involvement of IL-18 with the overproduction of IFN-γ and the inability of NK cells and cytolytic CD8+ T cells to lyse infected and activated antigen-presenting cells. Prolonged interaction between innate and adaptive immune cells will amplify a cascade of proinflammatory cytokines, which will stimulate macrophages in the process of hemophagocytosis and multisystem dysfunction
[43]. It has been observed that in MAS, the serum concentration of IL-18 is decisive for the amplification and perpetuation of the degree of activation of the cells of the innate immune system, and its plasma level can differentiate between sJIA complicated by MAS, and other inherited (monogenic) periodic fever syndromes
[44]. As already reported, IL-18 is known to be monitored and regulated by its high-affinity natural antagonist IL-18BP, which can block its biological activity. Although both entities are under the influence of inflammatory cytokines in sJIA and MAS, as well as in viral infections or various HLH-associated diseases, a major imbalance was detected with abnormal growth of bioactive IL-18, which appears to induce IFN-γ synthesis and, consequently, the MAS emergence
[45][46]. Lack of proportion in IL-18/IL-18BP concentrations gives rise to high systemic free bioactive IL-18, putting at risk MAS progress. The use of recombinant IL-18BP in patients with AOSD and sJIA with MAS has had encouraging effects for the application as useful biomarkers of IL-18, especially free IL-18, but also as attractive new medicines. Recent findings have shown that elevated levels of free IL-18 are present in correlation with clinical and biological signs of disease activity. A very interesting aspect is that some patients with these diseases have responded very well to treatment with recombinant human IL-18BP, which demonstrates the pathogenic role of Il-18 and recommends the use of IL-18 inhibitors in difficult-to-treat autoinflammatory diseases. In spite of the fact that the whole picture of MAS evolution is not fully comprehensible, the counterbalancing of level IL-18/IFN-γ in the management of severe HLH and MAS stopping could be a very good solution
[47][48].
Quite surprisingly, in the first wave of SARS-CoV-2 infection in China, the clinical parameters were identical to those observed in CSS. Despite the fact that CSS has been known for over a century, it is only in the last three decades that the scientific medical world has begun to deepen and unravel the clinical and biological aspects, its name covering as an umbrella HLH, the hemophagocytic syndrome associated with infection, the cytokine release syndrome, cytokine storm and MAS. During the COVID-19 pandemic, the appearance of cases with a brusque and fast evolution towards exitus, motivated the amplification of studies on pathogenesis, entity recognition and the adequate therapy of lethal CSS
[49].
Compared to SARS-CoV, which is thought to induce inadequate interferon (IFN) responses, SARS-CoV-2 robustly triggered the expression of numerous IFN-stimulated genes involved in inflammation
[50].
If researchers make a comparison between MAS and the occurrence of COVID-19 CSS, researchers can say that when the human body faces a viral infection, the virus-specific cytotoxic T lymphocytes (CTLs) get involved, working together with antigen-presenting cells (APC) and macrophages for stopping and removing viral agents. Physiologically, when the process is well completed, all the activated immune cells are removed by the cytolytic cells and the immune system, so that everything returns to the initial stage of prevention. In the process of activating macrophages, the innate immune defense system in teamwork with IL-18 trains APC and the macrophages to fight. If the process of activating innate immune cells and inadequate cytolytic cells persists, then the potency of removing the activated immune cells decreases, leading to long-lasting action of CTL-APC, and the permanent expulsion of inflammatory mediators, disruption of CTL-macrophage activity and the occurrence of such a cytokine storm as MAS. In SARS-CoV-2 infection, the same antiviral immune pathways will be activated with the extended participation of CTL. Participation of type I interferon will allow the persistence of lung infection, and the activation of type II interferon will facilitate the persistent process of immune activation. If the virus cannot be eliminated, there will be a continuous signaling from type I interferon, which will result in the production of proinflammatory cytokines. Similar to in MAS, the cytolytic capacity of CLT will be disrupted due to immune depletion and/or inappropriate antiviral replication, which would maintain a prolonged disagreement between CTL, APC and the alveolar macrophages, facilitating COVID-CS
[51].
It has been shown that both in MAS and HLH, but especially in severe COVID-19, it is implicated the dysfunction of type I interferon, NK cells, with extensive CTL involvement, increased IL-6, TNF-α, and excessive inflammation caused by the NF-kB pathway. Despite this similarity between the three prototypes of pathologies, there are distinct clinical features not found in MAS. According to several recent studies, severe COVID-19 is characterized in particular by clinical and biological signs of dramatic coagulopathy with complement activation, vascular endothelial lesions and microthrombosis, a pathology similar to thrombotic microangiopathy
[52][53][54].
Type I interferons have a very important role in innate immunity because toll-like receptors 3, 7 and 9 (TLR3, 7 and 9) are expressed
[55][56] as pattern recognition receptors (PRRs) on the surface of antigen-presenting cells (macrophages, dendritic cells) and B lymphocytes, that once activated, will express IFN, stimulate signaling pathways involved in NF-kB activation and interferon regulatory factors (IRF) IRF3 and IRF7, which will create the possibility of transcription and production of IFN-α and other proinflammatory cytokines
[57].
PRRs recognize both PAMPs and DAMPs derived from tissue damage, while type I IFNs (IFN-α and IFN-β) released from the cell establish an autocrine and paracrine loop with other molecules for the antiviral defense of the infected cell. If viral agents have not been removed, then the proinflammatory state intensifies, the cellular functions are disrupted, recruitment is intensified, and the activation of adaptive effector cells and release of a massive amount of proinflammatory factors take place.
Disruption of type I interferon activity by genetic mutations or by the involvement of some autoantibodies results in failure to stop the spread of primary SARS-CoV-2 infection in the lungs, especially the invasion of the alveoli by macrophages, which, when activated, maintain the immune processes
[58][59].
In patients with severe COVID-19, a strong activation of alveolar macrophages and very high pulmonary concentrations of proinflammatory mediators (IL-6, IL-8, IL-1β) and chemokines were found. In the early stage of SARS-CoV-2 infection, IFNs strongly participate in the antiviral activity by training NK cells and antigen-presenting dendritic cells, activating and differentiating virus-specific T cells, attracting memory T cells, T and B lymphocytes to the site of the impact. IFNs work antivirally by activating CTLs, inhibiting viral mRNA translation, disintegrating the viral RNA, and adjusting the nitric oxide release. Prolonged involvement of interferon to eliminate the virus facilitates the development of a self-sustaining outbreak in the lungs, which can be exacerbated by a feedback loop and will lead to a cytokine storm. Evidence of the involvement of IFNs in SARS-CoV-2 infection and recent advances in understanding the mechanisms of action have led to the development of targeted antiviral therapy with IFN-α and IFN-β. Research shows that the use of IFNs in the treatment of the early stages of the disease, more precisely before the onset of severe signs of inflammation, could be of great protective benefit to patients with COVID-19
[60][61][62].
Adachi et al. reviewed the publications using PubMed and Google Scholar for citations published after the outbreak of COVID-19, from December 2019 to July 2021, and identified six cases of JIA with COVID-19 during treatment with biologic DMARDs (anti-TNF-α or anti-IL-1β). Of the studied cases, only one patient with sJIA receiving oral prednisolone in combination with canakinumab required oxygen therapy for COVID pneumonia. The authors also presented a case of sJIA with MAS in remission for 5 years from the initiation of TCZ therapy at a dose of 8 mg/kg every 4 weeks and MTX (7 mg/m
2) weekly, personally studied. The patient developed a fever for two days and was diagnosed by way of a positive RT-PCR test with the SARS-CoV-2 α-variant, as was her father. At hospital admission, there were no signs of respiratory failure, chest X-ray abnormalities, or altered biological parameters, except for the cytokines TNF-α, IL-6 and IL-8 being slightly increased. The patient continued to receive oral MTX without any antiviral drugs or oxygen therapy, and TCZ was reintroduced after the fever disappeared. The case presented by the authors is the first with SARS-CoV-2 infection with the α-variant under treatment with TCZ. At discharge, the disease was controlled, and from a biological point of view, only IL-6 was detected at a low level. In the end, the authors mentioned that the COVID-19 disease was cured without serious complications in all forms of JIA under biologics, but not with long steroid therapy. These data support the hypothesis that patients with active JIA under steroid immunosuppressive therapy have a risk of developing severe COVID-19
[63][64][65].
Data from the literature converges to the idea that low-dose steroid immunosuppressive therapy in patients with JIA under biologic therapy does not significantly increase the risk of severe COVID-19. On the other hand, pediatric rheumatology studies indicate that MIS-C associated with severe COVID-19 has pathophysiological similarities to hyperinflammatory abnormalities in MAS in patients with sJIA treated with TCZ. At the same time, TCZ used in patients with sJIA may be useful in severe COVID-19 pneumonia
[66].
In a recent study, Aydın et al. investigated 13 patients with sJIA and MAS and 26 with MIS-C. The authors observed that patients with MAS had lower hemoglobin (10.10 g/dL) and fibrinogen (2.72 g/dL), but higher ferritin (17,863 mg/dL) and LDH (890.61 U/L) at diagnosis. Absolutely higher neutrophil counts (12,180/mm
3) and CRP values (194.23 mg/dL) were found in patients with MIS-C, while absolute lower lymphocyte count (1140/mm
3) were found at the time of diagnosis. The left ventricular ejection fraction was reduced in the MIS-C group. In conclusion, the authors showed that the levels of ferritin, hemoglobin, LDH and fibrinogen were significantly increased in MAS compared to MIS-C. However, patients with MIS-C had more severe signs of heart damage than those with MAS
[67].
3. Interrelationship between SARS-CoV-2 Infection and sJIA
Effects of the ongoing coronavirus pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with symptoms ranging from none to life-threatening, have also dramatically affected the rheumatic care, the initial presentation and the research on sJIA worldwide.
Dushnicky et al. collected data (the time from the onset of symptoms to the first assessment, the severity of disease on presentation and the recruitment record) from the archive of the Canadian Alliance of Pediatric Rheumatology Investigators (CAPRI) for JIA in the pre-pandemic year (11 March 2019–10 March 2020) and compared them with data from the first year of the pandemic (11 March 2020–10 March 2021), through appropriate statistical processing. Although the mean times from the onset of disease to the first presentation were comparable, and the frequency of different categories of JIA remained stable, sJIA was an exception (12 pre-pandemic cases, and only 1 in the pandemic). Otherwise, the clinical features, disease activity, disability scores and quality of life were similar. Authors concluded that in Canada, the COVID-19 pandemic did not affect the presentation to pediatric rheumatologists of new patients with JIA due to the pre-existing telemedicine infrastructure, which prioritized virtual over in-person consultations, adapted to new circumstances
[68].
The main objective of pediatric rheumatologists was to prevent the exacerbation of systemic autoinflammatory and/or autoimmune diseases in patients on chronic immunosuppressive therapies at risk of contracting, (and possibly) a more severe course of SARS-CoV-2 infection. All research has pointed out that to prevent a severe course of infection in rheumatic patients, vaccination should be widely recommended, being safe and effective
[69].
The imminent danger of very severe COVID-19 has been shown to vary with age, sex and the presence of comorbidities, so that it increases exponentially with age; with a two-fold increase in a single comorbidity, or five-fold greater threat in multimorbidity (three or more comorbidities)
[70].
Nearly all children with COVID-19 had mild symptoms and favorable evolution, except for MIS-C, a life-threatening illness and long-term sequelae
[28][71][72].
Walters et al. investigated the seroprevalence of SARS-CoV-2 IgG and the clinical course in a big group of pediatric patients with rheumatic diseases. The majority of positive subjects had no symptoms and the symptomatic ones all had mild COVID-19 symptoms, suggesting the lowest risk of severe or critical COVID-19 in immunocompromised rheumatic pediatric patients
[73].
To better guide clinicians when experiencing SARS-CoV-2 infection in pediatric rheumatic patients under immunomodulatory drugs or underlying diseases, Sozeri et al. investigated in a multicenter retrospective design, the clinical manifestations and the evolution of COVID-19 in 113 children receiving biologic disease-modifying antirheumatic drugs (DMARDs) due to their major rheumatic disorders. They were aged 12.87 ± 4.69 years and diagnosed as follows: 63 patients with JIA, 35 with systemic autoinflammatory diseases, 10 with vasculitis, and 5 cases of connective tissue disease. The mean duration of primary disease was 4.62 ± 3.65 years. Fever and dyspnea were considered risk factors for hospitalization, occurring in 21.2% of cases with a younger age, a shorter duration of the disease, and a higher rate of steroid application compared to outpatients (78.8%). The link between comorbidities (cardiovascular, hepatic, renal or malignant diseases) and a severe course of SARS-CoV-2 infection has been explored, however, is still limited for patients using biologic DMARDs. The authors concluded that there was no exacerbation of both COVID-19 and rheumatic disease under biologic DMARDs, but multicenter international studies are still needed to highlight all the risk factors for a severe course of COVID-19 among pediatric rheumatic patients
[74].
As the most common chronic rheumatic disease in children, JIA has raised the question of whether these patients are at higher risk and whether their treatment should be changed for COVID-19 infection.
Boyarchuk et al. investigated the frequency of COVID-19 infection in JIA children and their course in the circumstances of applied immunosuppressive therapy. Out of 51 patients with JIA, 10 (19.6%) patients had a SARS-CoV-2 infection which was most common in patients with sJIA, but with a similar course to the general pediatric segment, despite immunosuppressive therapy. In 3 out of 10 children, the infection exacerbated JIA, and an increase in therapy was imperative. Children with JIA and COVID-19 should be monitored to identify eventual long-run exacerbations after infection
[75].
Since children and teens have not experienced very often and generally only mild forms of SARS-CoV-2 infection compared to adults, Sengler et al. aimed to study the gravity, the clinical features and the course of COVID-19 in pediatric patients under diverse therapies for their primary rheumatic and musculoskeletal disease (RMD), using a survey within the German national pediatric rheumatology database. Due to corrective suppression of the immune responses, these subjects may be endangered of an extreme evolution of SARS-CoV-2 infection, or a recurrence or intensification of the rheumatic disease triggered by COVID-19. Seventy-six patients with RMD and positive for SARS-CoV-2 infection (mean age 14 years), of whom only fifty-eight showed symptoms, including three patients with sJIA, all symptomatic, had a mild course of disease with good progression, i.e., in the vast majority (84%) no significant increase in RMD activity was observed, regardless of whether the immunosuppressive medication was kept or discontinued. Only two patients were hospitalized, one of whom required intensive care and died of cardio-respiratory failure. The authors concluded that COVID-19 does not appear to have a substantial impact on RMD activity in children, but the clinical features, severity and outcome in patients with RMD under various therapies are not yet easy to understand
[76].
Finding a cure for COVID-19 was the most important life-saving task right from the start, and several vaccines have been developed, but none are 100% effective
[77].
Parums D.V. analyzed the evolution of the pandemic and the new mutations that have emerged, which have shown that both vaccinated and unvaccinated, especially people with a compromised immune system, will continue being infected with SARS-CoV-2. For this reason, it has been a matter of the utmost importance for research to advance therapies that minimize the gravity of the infection in those who suffer and need hospitalization. Because IL-6 is a proinflammatory cytokine whose serum concentration is high in COVID-19, a humanized monoclonal antibody to the IL-6 receptor (IL-6R), TCZ, was granted an emergency authorization in June 2021 by the FDA, to treat inpatients with moderate and severe COVID-19, based on the results of two clinical trials, REMAP-CAP (NCT02735707) and RECOVERY (NCT04381936). TCZ has been authorized by the FDA since 2011 for the treatment of rheumatoid arthritis, JIA and other autoimmune and autoinflammatory diseases. The use of anti-SARS-CoV-2 monoclonal antibodies is now in clinical progress, being tested in clinical trials worldwide, even as combined antibodies to treat moderate and severe infections. New reliability and output data on IL-6 and IL-6R targeting will emerge to implement more individualized therapies that work better to control the systemic impact of COVID-19
[78].
Moreover, the European Medicines Agency (EMA) has recently approved Actemra/Roactemra (tocilizumab) and recommended it against severe forms of COVID-19. This drug is also an option to combat cytokine storms or exaggerated immune reactions induced by certain cellular treatments
[79].
Because IL-6 plays a critical role in protecting the host from infections and tissue damage, and could serve as a valuable bioindicator in several specific cytokine storms, Kang et al. reviewed the present perspective on the multifarious roles of IL-6, receptors, and signaling pathways during severe inflammation which affects the whole body. IL-6 triggers the generation of various cytokines and chemokines by vascular endothelial cells, setting in motion the coagulation cascade, i.e., the endothelial cell disorder, featured by atypical coagulation and vascular leakage—an ordinary aggravating reaction in cytokine storms. Redundant IL-6 generation would conduct to chronic inflammatory diseases and hyperinflammation, such as cytokine storms. The authors point out that it has become increasingly clear that TCZ can successfully fulfill its mission of stopping IL-6 signaling with beneficial effects, not only in the management of cytokine storms in sJIA but also in other autoimmune and autoinflammatory disorders, including in sepsis and severe SARS-CoV-2 infection. Thus, IL-6, as a relevant pointer to cytokine storms, has been shown to control a variety of distinctive features linked to vascular homeostasis and inflammation. Blocking IL-6 signaling could be a helpful clinical master plan for different inflammatory conditions and induced cytokine storms. Regarding the interpretation of COVID-19 pathological studies, the use of TCZ has been established to be favorable in critically ill patients
[80].
In the COVID-19 pandemic, other interleukin inhibitors, for example, anakinra—a recombinant humanized IL-1 receptor antagonist—may also ameliorate the effects of hyperinflammation, cytokine-associated storms (characterized by a life-threatening, fulminant hypercytokinaemia with high mortality), or lately, identified MIS-C in pediatric patients
[81][82].
Phadke et al. investigated the intravenous administration of anakinra in the management of MAS secondary to sJIA, SLE or secondary hemophagocytic lymphohistiocytosis (sec-HLH) and others in 19 patients with a mean age of 13 years, indicating a possible positive effect in MAS/HLH critical cases and silencing of COVID-19-associated CSS or related MIS-C
[83].
A hope for patients with JIA who do not respond to biologics, or to potentiate it for better results, is photobiomodulation therapy, which creatively applied opens new avenues for quantified regulation of the immune system [84][85][86], sometimes avoiding the emergent adverse reactions to bDMARDs, for example sJIA-LD, a novel parenchymal lung disease, increasingly detected in sJIA [87][88].
The comparative representation of the cytokine storm picture in sJIA-MAS and COVID-19 CS with the multiple organ dysfunctions in both pathologies is highlighted in Figure 1.
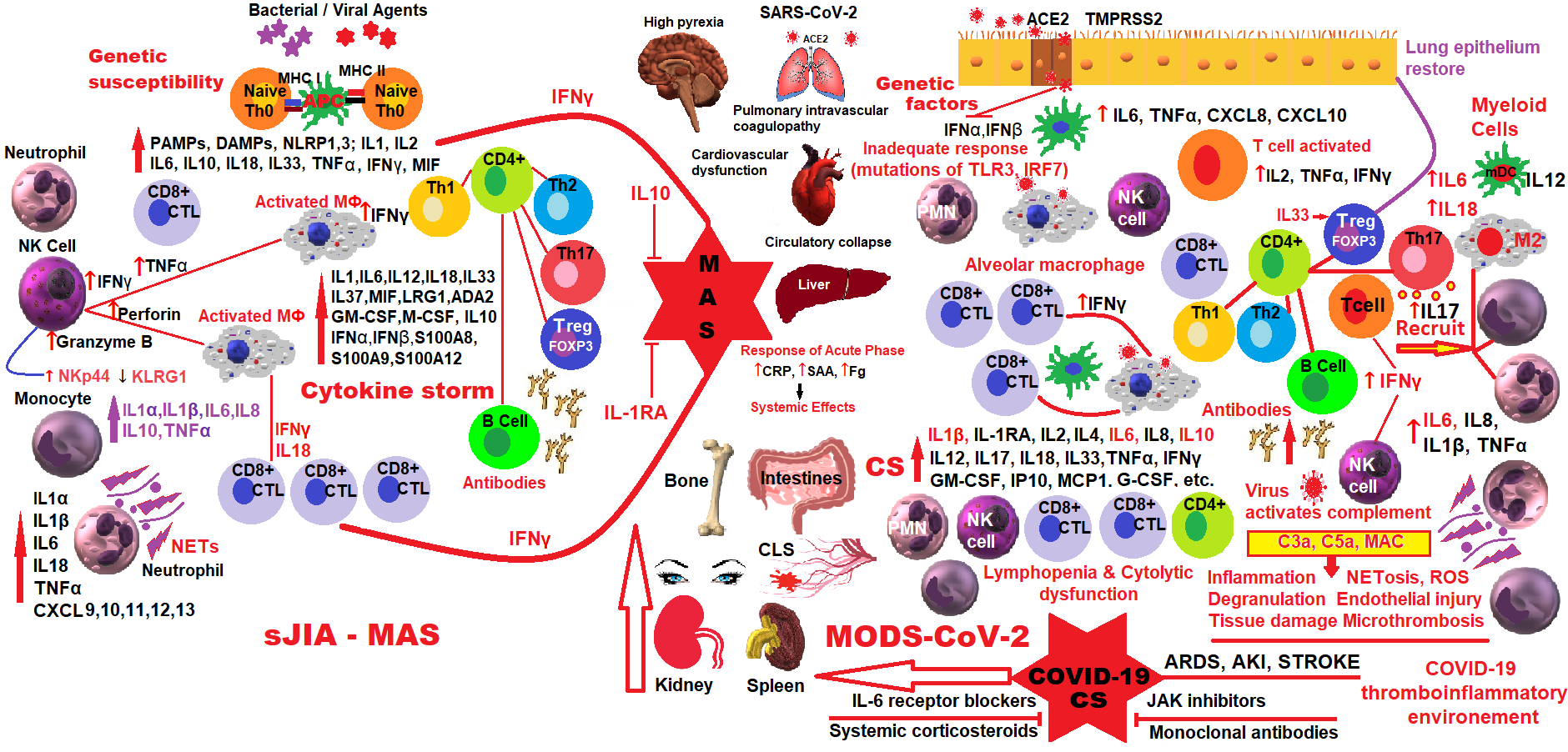
Figure 1. Complex cytokine storms in sJIA-MAS and COVID-19-CS and secondary organ dysfunction (the figure was imagined and drawn by L.M.A. using Microsoft Paint 3D (3D Library - Biology: human heart and brain) for Windows 10 and using also completely free picture material (human lungs, kidney, eyes, intestines, CLS and bone cliparts) from SeekPNG.com, for which researchers are very grateful).
4. Conclusions
COVID era has shown that there are serious cases in which the patient's antiviral overregulated response becomes harmful by itself, as in sJIA-MAS. This entry draws attention to the fact that the failures in the treatment of MAS in sJIA patients, and those with severe COVID-19 could be due to a misunderstanding of the two different and opposed MAS immunopathologies - one with loss of immune function, and the other with gain of immune function. It is considered that MAS reaches its highest point through a multiple interactive network of infectious, genetic, innate immune deregulated factors, that could generate distinct conditions, such as sepsis, sJIA, AOSD, cancer etc.
It turned out that MAS is in a complex relationship with infectious diseases, insufficiently well defined, and in the case of COVID-19, MAS manifests itself as a distinct phenotype, for which researchers do not yet have a complete description.
As there is still a risk of new mutations in the SARS-CoV-2 virus, measures are needed to educate families, children, teachers and civil society to better cope with the impact of COVID-19 and the new challenges. Researchers, medical doctors and especially pediatric rheumatologists need to develop new integrative diagnostic and treatment approaches to further support children with RMDs and COVID-19.
Multicenter randomized controlled trials are still acutely necessary to establish at what time and by what means should immunoregulatory products be administered in sJIA-MAS patients with negative response to corticosteroids or contraindications.

