Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Virus filtration is a single-use, size-based separation process in which the contaminating virus particles are retained while the therapeutic molecules pass through the membrane pores. Virus filtration is routinely used as part of the overall virus clearance strategy. Compromised performance of virus filters due to membrane fouling, low throughput and reduced viral clearance, is of considerable industrial significance and is frequently a major challenge.
- process development
- virus filtration
1. Introduction
A virus filtration step is frequently included to provide a robust size-based clearance of both enveloped and non-enveloped viruses during the manufacture of mammalian cell-derived biotherapeutics, such as monoclonal antibodies (mAbs) and Fc-fusion proteins [1][2]. Before approval of new therapeutics, regulatory agencies such as the US Food and Drug Administration (FDA) require validation of adequate virus clearance [3]. Consequently, unit operations are added to the purification train to ensure high levels of virus clearance [4]. Virus filtration uses large pore size ultrafiltration membranes to retain any contaminating virus particles while recovering the virus-free product in the permeate. Unlike conventional ultrafiltration operations, the performance criteria for virus filters are far stricter [5]. Typically, around 95% product recovery is required while maintaining at least 1000 fold (3 log reduction) virus clearance [2].
Table 1 lists a range of mammalian cell-derived biotherapeutics that have been approved by the Food and Drug Administration (FDA) over the last three decades. The monoclonal antibody industry sector grossed over USD 154 billion in 2020 [6][7]. Mammalian cells used for expression of recent FDA-approved monoclonal antibodies include Chinese Hamster Ovary (CHO) cells and murine myeloma cells (Sp2/0, NS0), among others [6][8].
| Drug Classification | Examples | First Approval by FDA | Manufacturer |
|---|---|---|---|
| Monoclonal antibodies | Pembrolizumab | 2014 | Merck |
| Nivolumab | 2014 | Bristol Myers Squibb | |
| Aducanumab | 2021 | Biogen | |
| Avelumab | 2017 | EMD Serono | |
| Omalizumab | 2003 | Genentech | |
| Adalimumab | 2002 | Abbvie | |
| Tezepelumab-ekko | 2021 | Amgen/AstraZeneca | |
| Fc-fusion proteins | Abatacept | 2021 | Bristol Myers Squibb |
| Aflibercept | 2011 | Regeneron | |
| Alefacept | 2003 | Biogen | |
| Etanercept | 1998 | Amgen | |
| Rilonacept | 2008 | Regeneron | |
| Cytokines | Darbepoetin alfa | 2011 | Amgen |
| Interferon beta-1a | 2003 | Biogen | |
| Epoetin alfa | 2011 | Amgen | |
| Enzymes | Agalsidase beta | 2003 | Genzyme |
| Human DNase | 1993 | Genentech | |
| Laronidase | 2003 | Biomarin | |
| Tenecteplase | 2000 | Genentech | |
| Hormones | Choriogonadotropin alfa | 2000 | EMD Serono |
| Follitropin alfa | 2004 | EMD Serono | |
| Osteogenic protein-1 | 2001 | Stryker Biotech | |
| Thyrotropin alfa | 1998 | Genzyme |
Table 1. Examples of approved Chinese Hamster Ovary (CHO) cell-derived biotherapeutics. Non-exhaustive list compiled from publicly available resources (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process), US Food and Drug Administration, (last accessed 10 January 2022), European Medicines Agency [9].
Virus filtration is different from typical pressure-driven membrane filtration processes, as the filter is designed to obtain very high levels of removal of potential virus contaminants. Further, as it is impractical to validate that there is zero carryover of any trapped virus particles, reuse of the virus filter is impossible. Consequently, these are single-use devices. Virus filters are typically run in normal flow (dead end) mode, rather than tangential flow mode used for protein ultrafiltration, since normal flow is less complex and requires only a single pump.
2. Downstream Processing
2.1. Platform Processes
Biopharmaceutical manufacturing processes can be divided into two main processing trains: upstream cell culture operations and downstream purification processes. Various bioreactor configurations are used to produce the cells that express the product of interest (mAbs, enzymes, Fc-fusion proteins, or hormones). Removing particulate matter such as cells and cell debris occurs at the interface between upstream and downstream unit operations. These bioreactor clarification operations are sometimes referred to as midstream processes [10][11].
Figure 1 is a typical ‘platform’ process for the downstream purification of monoclonal antibodies. The first unit operation is typically an affinity chromatography capture step using protein A (resin-based chromatography) [12]. Affinity interaction is a specific interaction based on both the topological fit and a combination of electrostatic, hydrophobic, and hydrogen-bonding interactions [13]. Antibody elution from the protein A column is performed at low pH, making it very convenient to include a low pH hold for virus inactivation.
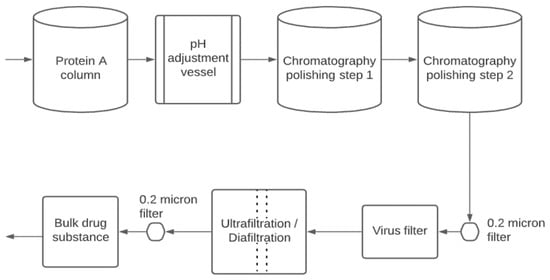
Figure 1. Downstream purification of mammalian cell-derived biotherapeutics.
Frequently, two polishing steps are used to remove the remaining impurities and product variants/aggregates [14]. Resin- or membrane-based chromatography (ion exchange or hydrophobic interaction chromatography) is frequently used. The polishing steps remove impurities such as DNA, host cell proteins (HCP), and product aggregates [2]. Typically, all streams and buffers which enter the purification process are passed through sterilizing grade (0.22 μm pore size) filters to reduce bioburden.
As shown in Figure 1, the virus filtration step is typically located near the end of the purification train. The product is relatively concentrated and highly purified. High product concentrations can lead to compromised performance due to product aggregation and increased adsorption to the virus filter membrane. A final ultrafiltration/diafiltration step is used to concentrate the product and place it in the formulation buffer needed for stability during shipping/storage and delivery to the patient. The final 0.22 µm pore size filter is used to ensure sterility of the product and is often part of the final fill-finish operation.
2.2. Viruses, Virus Clearance, and Virus Filters
Many mammalian cell lines produce endogenous retrovirus-like particles [2]. These particles are typically around 80–100 nm in size. Clearance can be achieved by inactivation and/or physical removal from the process stream [15][16][17]. During purification, manufacturers of mammalian cell-derived biotherapeutics must demonstrate that the process will yield a final product containing no more than one virus particle in a million doses. Estimates of the number of virus particles in a single dose equivalent from the bioreactor could be as high as 1010–1015 retrovirus-like particles per mL [2]. Removal of adventitious viruses such as parvovirus is also required. These much smaller viruses are around 20 nm in size. In the past, filters targeted for retrovirus and parvovirus removal were included in the purification train [3]. Recent studies show that virus clearance filters designed to provide clearance of smaller parvovirus can be used to clear much larger retroviruses simultaneously [18].
Table 2 shows some viruses that are employed for validation studies in biomanufacturing. The enveloped retroviruses are typically larger than the non-enveloped parvoviruses. Consequently, virus filtration membranes that are validated for removal of parvovirus are also effective at clearing retrovirus from the product.
Table 2. Some common viruses used for validation studies in biomanufacturing [19].
| Name of Virus | Diameter (nm) |
|---|---|
| Animal parvoviruses (non-enveloped DNA viruses, bovine, canine, or porcine) | 18–24 |
| Poliovirus (picornavirus, non-enveloped RNA virus) | 25–30 |
| Encephalomyocarditis virus (EMC, picornavirus, non-enveloped RNA virus) | 25–30 |
| Feline calicivirus (calicivirus, non-enveloped RNA virus) | 35–39 |
| Bovine viral diarrhea virus (BVDV, flavivirus, enveloped RNA virus) | 40–60 |
| SV40 (simian vacuolating virus 40, polyomavirus, non-enveloped DNA virus) | 45–55 |
| Sindbis virus (togavirus, enveloped RNA virus) | 60–70 |
| Reovirus (non-enveloped RNA virus) | 60–80 |
| Herpes simplex virus (HSV, Herpesviridae, enveloped DNA virus) | 150 |
| Pseudorabies virus (PRV, Herpesviridae, enveloped DNA virus) | 120–200 |
Adventitious virus contamination is a concern in the manufacture of biologics. Validation of virus clearance is shown by conducting scale-down testing [20]. The feed is spiked with model virus particles, and clearance in the product stream is determined. Minute virus of mice (MVM, mouse parvovirus) is often used to validate adventitious virus clearance. The FDA requires at least two orthogonal steps with different mechanisms of action for validation of virus clearance with the required level of virus clearance for the process as a whole, determined by summing the clearances obtained from the individual unit operations [2].
Virus filtration uses porous polymeric membranes in normal flow mode [15][21][22]. The predominant mechanism of action for virus filters is size exclusion [15]. The difference in hydrodynamic diameter between a protein product and MVM is often less than two-fold [5]. Today, virus filters are a critical component of the overall virus clearance strategy [15]. As shown in Table 3, virus filter membranes are typically made of regenerated cellulose, polyvinylidene difluoride (PVDF), and polyethersulfone. The latter two materials are hydrophilized in order to minimize fouling by adsorption and maximize flux during virus filtration. While the membrane should be biocompatible, non-fouling, and minimize adsorption on the membrane surface, it is also essential that the membrane is robust and dimensionally stable to ensure the required level of virus clearance.
Table 3. Commercially available virus filters [2][23][24]. Asahi Kasei Bioprocess is a part of the Asahi Kasei Group; MilliporeSigma is a subsidiary of Merck KGaA.
| Filter | Manufacturer | Membrane Material | Configuration | Comments |
|---|---|---|---|---|
| Planova 15 N, 20 N | Asahi Kasei Bioprocess | Regenerated cellulose | Asymmetric single-layer hollow fibers | Parvovirus filter |
| Planova 35 N | Asahi Kasei Bioprocess | Regenerated cellulose | Asymmetric single-layer hollow fibers | Retrovirus filter |
| Planova BioEX | Asahi Kasei Bioprocess | Hydrophilized PVDF | Asymmetric single-layer hollow fibers | Parvovirus filter |
| Viresolve NFR | MilliporeSigma | Polyethersulfone | Asymmetric triple-layer pleated sheets | Retrovirus filter |
| Viresolve Pro | MilliporeSigma | Polyethersulfone | Asymmetric double-layer flat sheets | Parvovirus filter |
| Pegasus SV4 | Pall Corporation | Hydrophilized PVDF | Symmetric double-layer pleated sheets | Parvovirus filter |
| Pegasus Prime | Pall Corporation | Polyethersulfone | Pleated sheets | Parvovirus filter |
| Ultipor VF DV20 | Pall Corporation | Hydrophilized PVDF | Symmetric double-layer pleated sheets | Parvovirus filter |
| Ultipor VF DV50 | Pall Corporation | Hydrophilized PVDF | Symmetric double-layer pleated sheets | Retrovirus filter |
| Virosart HC | Sartorius AG | Polyethersulfone | Asymmetric double-layer pleated sheets | Parvovirus filter |
| Virosart HF | Sartorius AG | Modified polyethersulfone | Asymmetric single-layer hollow fibers | Parvovirus filter |
These virus filters are designed to ensure that only monomeric biomolecules with a hydrodynamic diameter less than 20 nm can pass through the pores. Much research is needed to understand how a multidomain, anisotropic mAb with varied surface moieties interacts with virus filtration membranes, prefilters, and other product monomers [25].
Table 3 is a non-exhaustive list showing commercially available virus filters and material configurations. Operating pressures and permeate fluxes vary greatly. Virus filter membrane fouling is a significant challenge [22][26][27]. Fouling can compromise virus clearance and reduce membrane productivity (product recovered per membrane surface area) [28]. Fouling is often due to product variants because of the high product purity before virus filtration and the high product concentration compared to the spiked virus concentration [2].
Recent studies focusing on virus filtration of mAbs showed that membrane performance depends on the mAb properties (pI, hydrophobicity, net charge, dipole moment, oligomericity), buffer conditions, membrane material, and operating pressure [22][29]. Buffer excipients such as arginine and lysine can stabilize mAbs and reduce fouling propensities [30]. Excipients such as histidine, arginine, and lysine can reduce reversible self-association of mAbs to varying degrees [31]. Reversible self-association is often concentration-dependent [31].
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering9040155
References
- Troccoli, N.M.; McIver, J.; Losikoff, A.; Poiley, J. Removal of viruses from human intravenous immune globulin by 35 nm nanofiltration. Biologicals 1998, 26, 321–329.
- Wickramasinghe, S.R.; Namila; Fan, R.; Qian, X. Virus Removal and Virus Purification. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–96.
- FDA. Points to Consider in the Manufacture and Testing of Monoclonal Antibody Products for Human Use; Bethesda: Rockville, MD, USA, 1997.
- Han, B.; Carlson, J.O.; Powers, S.M.; Wickramasinghe, S.R. Enhanced virus removal by flocculation and microfiltration. Biotechnol. Bioprocess Eng. 2002, 7, 6–9.
- Billups, M.; Minervini, M.; Holstein, M.; Feroz, H.; Ranjan, S.; Hung, J.; Bao, H.; Ghose, S.; Li, Z.J.; Zydney, A.L. Antibody retention by virus filtration membranes: Polarization and sieving effects. J. Membr. Sci. 2021, 620, 118884.
- Dhara, V.G.; Naik, H.M.; Majewska, N.I.; Betenbaugh, M.J. Recombinant Antibody Production in CHO and NS0 Cells: Differences and Similarities. BioDrugs 2018, 32, 571–584.
- Global Market Insights. Monoclonal Antibodies Market Size By Type (Fully Human, Humanized, Chimeric), By Application (Oncology, Autoimmune Diseases, Infectious Diseases), By End-use (Hospitals, Clinics), COVID-19 Impact Analysis, Regional Outlook, Application Potential, Competitive Market Share & Forecast, 2021–2027. Available online: https://www.gminsights.com/industry-analysis/monoclonal-antibodies-market (accessed on 19 March 2022).
- Wagner, E.; Colas, O.; Chenu, S.; Goyon, A.; Murisier, A.; Cianferani, S.; Francois, Y.; Fekete, S.; Guillarme, D.; D’Atri, V.; et al. Determination of size variants by CE-SDS for approved therapeutic antibodies: Key implications of subclasses and light chain specificities. J. Pharm. Biomed. Anal. 2020, 184, 113166.
- Dumont, J.; Euwart, D.; Mei, B.; Estes, S.; Kshirsagar, R. Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Crit. Rev. Biotechnol. 2016, 36, 1110–1122.
- Zalai, D.; Kopp, J.; Kozma, B.; Küchler, M.; Herwig, C.; Kager, J. Microbial technologies for biotherapeutics production: Key tools for advanced biopharmaceutical process development and control. Drug Discov. Today Technol. 2020, 38, 9–24.
- DePalma, A. Continuity Promotes Bioprocessing Intensity. Genet. Eng. Biotechnol. News 2016, 36, 1–24.
- Vunnum, S.; Vedantham, G.; Hubbard, B. Protein A-Based Affinity Chromatography. In Process Scale Purification of Antibodies; Gottschalk, U., Ed.; Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 113–133.
- Bramer, C.; Tunnermann, L.; Gonzalez Salcedo, A.; Reif, O.W.; Solle, D.; Scheper, T.; Beutel, S. Membrane Adsorber for the Fast Purification of a Monoclonal Antibody Using Protein A Chromatography. Membranes 2019, 9, 159.
- Chollangi, S.; Parker, R.; Singh, N.; Li, Y.; Borys, M.; Li, Z. Development of robust antibody purification by optimizing protein-A chromatography in combination with precipitation methodologies. Biotechnol. Bioeng. 2015, 112, 2292–2304.
- Wickramasinghe, S.R.; Stump, E.D.; Grzenia, D.L.; Husson, S.M.; Pellegrino, J. Understanding virus filtration membrane performance. J. Membr. Sci. 2010, 365, 160–169.
- Huang, P.Y.; Peterson, J. Scaleup and virus clearance studies on virus filtration in monoclonal antibody manufacture. In Membrane Separations in Biotechnology; Marcel Dekker: New York, NY, USA, 2001.
- Bolton, G.R.; Spector, S.; LaCasse, D. Increasing the capacity of parvovirus-retentive membranes: Performance of the Viresolve™ Prefilter. Biotechnol. Appl. Biochem. 2006, 43, 55–63.
- Miesegaes, G.R.; Lute, S.C.; Read, E.K.; Brorson, K.A. Viral clearance by flow-through mode ion exchange columns and membrane adsorbers. Biotechnol. Prog. 2014, 30, 124–131.
- Burnouf, T.; Radosevich, M. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia 2003, 9, 24–37.
- Wieser, A.; Berting, A.; Medek, C.; Poelsler, G.; Kreil, T.R. The evolution of down-scale virus filtration equipment for virus clearance studies. Biotechnol. Bioeng. 2015, 112, 633–637.
- Kern, G.; Krishnan, M. Virus Removal by Filtration: Points to Consider. BioPharm Int. 2006, 19, 32–41.
- Bieberbach, M.; Kosiol, P.; Seay, A.; Bennecke, M.; Hansmann, B.; Hepbildikler, S.; Thom, V. Investigation of fouling mechanisms of virus filters during the filtration of protein solutions using a high throughput filtration screening device. Biotechnol. Prog. 2019, 35, e2776.
- Gefroh, E.; Dehghani, H.; McClure, M.; Connell-Crowley, L.; Vedantham, G. Use of MMV as a Single Worst-Case Model Virus in Viral Filter Validation Studies. PDA J. Pharm. Sci. Technol. 2014, 68, 297.
- Johnson, S.A.; Chen, S.; Bolton, G.; Chen, Q.; Lute, S.; Fisher, J.; Brorson, K. Virus filtration: A review of current and future practices in bioprocessing. Biotechnol Bioeng 2021, 119, 743–761.
- Robinson, J.; Roush, D.; Cramer, S. Domain contributions to antibody retention in multimodal chromatography systems. J. Chromatogr. A 2018, 1563, 89–98.
- Rayfield, W.J.; Roush, D.J.; Chmielowski, R.A.; Tugcu, N.; Barakat, S.; Cheung, J.K. Prediction of viral filtration performance of monoclonal antibodies based on biophysical properties of feed. Biotechnol. Prog. 2015, 31, 765–774.
- Bolton, G.R.; Basha, J.; Lacasse, D.P. Achieving high mass-throughput of therapeutic proteins through parvovirus retentive filters. Biotechnol. Prog. 2010, 26, 1671–1677.
- Kosiol, P.; Kahrs, C.; Thom, V.; Ulbricht, M.; Hansmann, B. Investigation of virus retention by size exclusion membranes under different flow regimes. Biotechnol. Prog. 2019, 35, e2747.
- Namila, F.N.U.; Zhang, D.; Traylor, S.; Nguyen, T.; Singh, N.; Wickramasinghe, R.; Qian, X. The effects of buffer condition on the fouling behavior of MVM virus filtration of an Fc-fusion protein. Biotechnol. Bioeng. 2019, 116, 2621–2631.
- Rodrigues, D.; Tanenbaum, L.M.; Thirumangalathu, R.; Somani, S.; Zhang, K.; Kumar, V.; Amin, K.; Thakkar, S.V. Product-Specific Impact of Viscosity Modulating Formulation Excipients During Ultra-High Concentration Biotherapeutics Drug Product Development. J. Pharm. Sci. 2021, 110, 1077–1082.
- Hu, Y.; Arora, J.; Joshi, S.B.; Esfandiary, R.; Middaugh, C.R.; Weis, D.D.; Volkin, D.B. Characterization of Excipient Effects on Reversible Self-Association, Backbone Flexibility, and Solution Properties of an IgG1 Monoclonal Antibody at High Concentrations: Part 1. J. Pharm. Sci. 2020, 109, 340–352.
This entry is offline, you can click here to edit this entry!
