Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physics, Condensed Matter
An Investigation of the Pressure-Induced Structural Phase
Transition of Nanocrystalline -CuMoO4
- high pressure
- phase transition
- synchrotron radiation
- X-ray diffraction
1. Introduction
Nanomaterials play an important role in catalysis, optics, electricity, and other research fields due to their exclusive and typical characteristics. In the past decades, compared to bulk materials, their nanocrystalline counterparts have gained massive attention due to their novel properties such as thermal, electronic, and magnetic, owing to their specific shape, size, and surface to volume ratio. In addition to that, nanomaterials also find their application in the fields of biology, medicine, and chemical industry [1,2,3,4]. Metal molybdates (AMO4) are important inorganic materials which have gained enormous scientific importance due to their wide range of applicability, such as industrial catalysts, photoluminescence, microwave applications, optical fibers, humidity sensors, scintillator materials and due to their magnetic and electrochemical properties [5,6,7,8].
Among molybdates, CuMoO4 is the compound with a very complex polymorphism [9,10,11,12]. Till date, six different polymorphs of CuMoO4 have been identified in the literature: namely ambient condition α-CuMoO4 [9], high temperature β-CuMoO4 [10], low temperature γ-CuMoO4 [11], high pressure (HP) CuMoO4-II [12], distorted wolframite CuMoO4-III [13] and monoclinic ε-CuMoO4 [14]. Earlier, single-crystal high-pressure and low-temperature X-ray diffraction studies carried by Wiesmann et al. [15] found that phase α-CuMoO4 undergoes a structural phase transition to γ-CuMoO4. This occurs during cooling at 190 K at ambient pressure as well as under increasing pressure at 0.2 GPa and room temperature. Congruently, high pressure and low temperature optical-absorption measurements carried out by Rodriguez et al. [16] found that α-CuMoO4 undergoes a first-order transition at 0.25 GPa to γ-CuMoO4. They found the same transition by cooling α-CuMoO4 to 200 K at ambient pressure. The α to γ phase transition involves a change of part of the copper coordination polyhedra, from square-pyramidal (C4v) CuO5 in α-CuMoO4 to octahedral elongated (D4h) CuO6 in γ-CuMoO4. The transition also involves a volume drop of 13%.
Many of the applications of CuMoO4 involve its use in the form of nanoparticles [17,18,19]. However, the information available on the structural and mechanical properties of nanocrystalline CuMoO4 is scarce. It is known that the high-pressure (HP) behavior of materials could be different for nanoparticles than for bulk materials [20,21,22,23]. In particular, transition pressures and compressibilities could be affected when the particle size is reduced [20,21,22,23]. To explore the structural and mechanical properties of nanocrystalline CuMoO4 under compression, the performance of an HP X-ray diffraction (XRD) investigation is needed. However, such studies have not been carried out yet in nanocrystalline α-CuMoO4.
2. Experiment
CuMoO4 nanoparticles were prepared following the method reported by Hassani et al. [20]. The chemical composition of the obtained CuMoO4 nanoparticles was determined by energy-dispersive X-ray spectrometry (EDXS), which was recorded with a JEOL JEM-6700F device. A EDXS spectrum is shown in Figure 1. The analysis showed that the composition of the nanoparticles was 67 (3) atomic % oxygen, 16 (1) atomic % copper, and 17 (1) atomic % molybdenum, which agrees with the stoichiometry of CuMoO4.
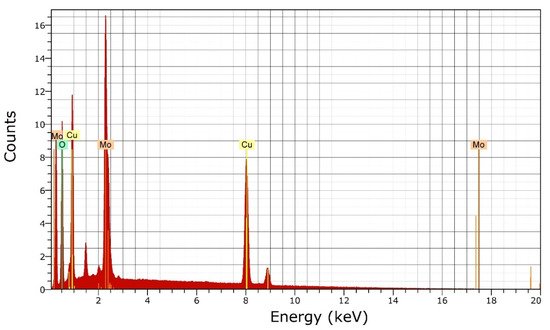
Figure 1. Energy dispersive X-ray analysis (EDXS) spectrum of nanocrystalline CuMoO4.
Ambient conditions powder XRD (λ = 0.4246 Å) performed in the same set up as high-pressure measurements confirmed that CuMoO4 nanoparticles crystallize in the triclinic α polymorph (space group P1¯
). The average particle size was estimated from powder XRD using the Scherrer equation [24], to be 43 (7) nm. This value was confirmed using MAUD [25], which gave an average particle size of 40 (9) nm. Angle-dispersive powder HP-XRD measurements were performed at the BL04-MSPD beamline of ALBA synchrotron [26]. An incident monochromatic X-ray beam with a wavelength of 0.4246 Å was focused down to a spot size of 20 μm × 20 μm (FWHM). Two-dimensional (2D) XRD data were recorded using a Rayonix SX165 CCD detector. The 2D images were integrated into one-dimensional intensity versus 2θ patterns using Dioptas [27]. The sample-to-detector distance along with detector parameters were determined using a LaB6 calibrant. A pellet of CuMoO4 nanocrystalline powder was loaded into a Boehler-Almax diamond-anvil cell (DAC) with diamond culets of 500 μm using a T301 stainless-steel gasket, pre-indented to a thickness of 50 μm and with a sample chamber of 200 μm diameter in the center. As pressure-transmitting medium, we used a 4:1 methanol–ethanol to allow a direct comparison with previous HP studies performed in microscopic samples [15]. The pressure medium can be considered nearly hydrostatic within the pressure limit of the experiments [28]. Special attention was paid to occupy only a minor fraction on the pressure chamber with sample to avoid sample bridging between diamonds [29]. The pressure was determined using ruby fluorescence [30]. Small ruby spheres with a grain size of ~1 μm and concentration of 3000 ppm Cr3+ [31] were used for pressure calibration [31] based on the wavelength shift of the R1 fluorescence band of the trivalent Cr3+ [32,33]. The estimated relative error for all measured pressures was better than 1% [34]. The unit-cell parameters were initially determined using the Le Bail analysis incorporated in the GSAS software [35]. Using the same software, Rietveld analysis [36] was also carried out for the initial α-CuMoO4 phase and high pressure γ-CuMoO4 phase.This entry is adapted from the peer-reviewed paper 10.3390/cryst12030365
This entry is offline, you can click here to edit this entry!
