Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Chitosan is the product of N-deacetylation of chitin. Due to the simultaneous presence of amino, acetamido, and hydroxyl groups in the molecule, chitosan is quite active in nature and can be modified, activated, and coupled, showing rich functionality and modifiability in biological applications. As the only polycationic polymer of natural origin, chitosan is capable of interacting with negatively charged cell membranes to assist the loading of drugs across the cell membrane.
- chitosan and its derivatives
- transdermal administration
- permeation promotion
- mechanism
- application
1. Introduction
The skin is composed of three parts, namely, the epidermis, dermis, and subcutaneous tissues from outside to inside. The epidermis is divided into the stratum corneum, transparent layer, granular layer, spinous layer, and basal layer from outside to inside [1][2][3]. The stratum corneum is located on the shallowest surface of the skin and is composed of multiple layers of dead keratinocytes. It has the effects of moisturizing, anti-friction, preventing the extravasation of tissue fluid from the body, and preventing the invasion of chemicals and bacteria into the body [4][5]. Designing the most appropriate delivery route for pharmaceutical preparations has long been an important research topic in the field of drug delivery. As the organ with the largest mass and surface area of the human body, the skin is undoubtedly the ideal delivery route for many drugs [6][7][8]. Compared with other administration routes such as intravenous administration and oral administration, skin administration has the advantage of avoiding the first-pass effect, long administration interval, stable blood concentration, realizing local or systemic administration, etc. [9][10], However, skin disorders represented by the stratum corneum largely limit the percutaneous absorption of many drugs. Therefore, how to achieve efficient transdermal drug delivery to achieve better therapeutic effects is the main challenge facing researchers.
The existing common transdermal preparations mainly comprise patches, ointments, spreads, sprays, and the like [11][12][13][14], These preparations are widely used for the treatment of skin disease. Laser devices in fractional mode have also been proposed in order to enhance the transdermal delivery of different drugs [15]. However, they have some disadvantages, including low transdermal penetration of drugs, low bioavailability, adverse reactions such as local stimulation of the skin, and poor compliance of patients [16][17][18][19][20]. Researchers are looking for various new auxiliary materials to address the above problems. Some synthetic high-molecular-weight polymers such as PEG and PLA have been used for transdermal drug delivery due to their excellent stability and adhesion properties [21][22][23][24][25]. However, the degradation inertness, cytotoxicity, and resulting immunogenicity of these synthetic high-molecular-weight polymers are problems that researchers must consider; accordingly, researchers tend to use high-molecular-weight polymers of natural origin such as polysaccharides. Polysaccharides are formed by condensation and dehydration of multiple monosaccharide molecules. They are carbohydrate substances with complex molecular structure and large size. Chitosan is a product of partially removing the acetyl of natural polysaccharide chitin, and it has multiple physiological functions such as inhibiting bacteria, resisting cancer, reducing blood fat, and enhancing immunity [26][27][28][29][30][31]. Chitosan and its derivatives have become a hot topic in research on transdermal drug delivery due to their good biocompatibility, biodegradability, and nontoxicity.
Chitosan is the product of N-deacetylation of chitin. Due to the simultaneous presence of amino, acetamido, and hydroxyl groups in the molecule, chitosan is quite active in nature and can be modified, activated, and coupled, showing rich functionality and modifiability in biological applications. As the only polycationic polymer of natural origin, chitosan is capable of interacting with negatively charged cell membranes to assist the loading of drugs across the cell membrane [32][33]. The polycationic nature of chitosan and its derivatives allows them to easily interact with the negative charge on the protein to facilitate protein drug encapsulation [34]. Chitosan and its derivatives can also chelate heavy-metal ions and are high-performance metal ion-trapping agents [35].
As mentioned above, chitosan is rich in active groups, which is conducive to achieving a variety of reactions to obtain chitosan derivatives with different characteristics. Carboxymethyl chitosan can be prepared through N-alkylation or O-alkylation, which shows improved water solubility and bacteriostasis and has good film-forming properties [36][37]. The N-acyl compound generated by the reaction of chitosan and glutamic acid has good moisture retention and adsorbability, and it can be used for absorbing metal ions [38]. The N-quaternary ammonium salt can be used for preparing polycationic polymers and enhancing the adhesion and adsorption capacity [39][40]. The product obtained by phosphorylation of chitosan can promote bone tissue regeneration and calcium absorption [41][42]. The chitosan sulfonation product has a heparinoid effect, has good bacteriostatic activity, and improves immunity [43][44][45]. These chitosan derivatives with different properties have been used for drug delivery research and have shown unique advantages. Figure 1 shows several major modification reactions of chitosan and the obtained products.
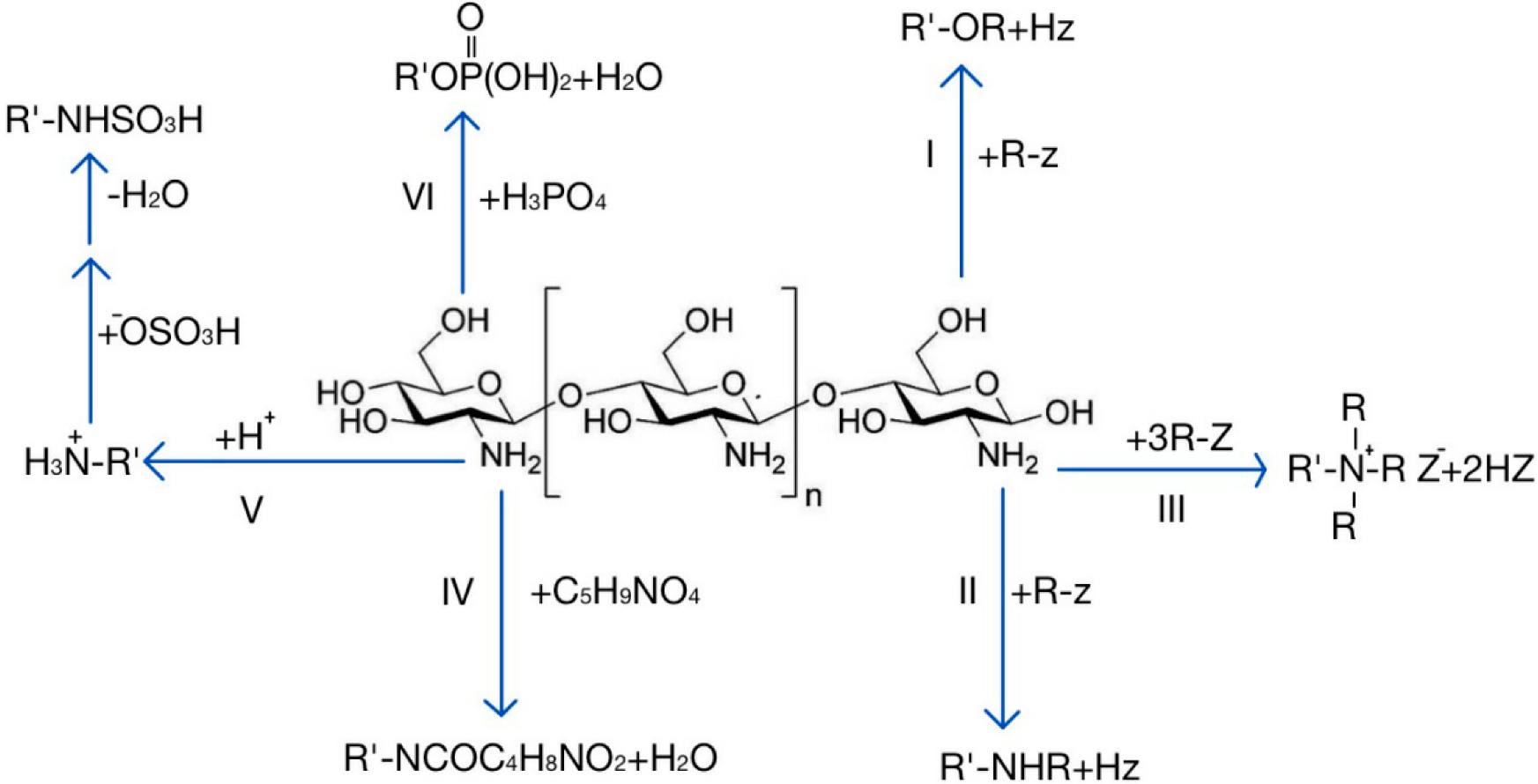
Figure 1. Several main modification reactions of chitosan. I: O-alkylation; II: N-alkylation; III: quaternization; IV: reaction with glutamic acid; V: sulfonation; VI: phosphorylation.
Chitosan and its derivatives can be degraded by physical methods [46][47][48], chemical methods [49][50], and enzymatic methods [51][52][53]. Chitosan involved in transdermal drug delivery is degraded in vivo by lysozymes, without the need of other reagents, and there is no generation of toxic byproducts in the reaction, thus representing the most ideal method of chitosan degradation. Lysozymes hydrolyze chitosan into low-molecular-weight, nontoxic chitosan oligosaccharides by acting on the β-1-4 glycosidic bond of the chitosan chain, and this degradation is not substrate-specific [54]; however, the enzymatic degradation of chitosan is related to its degree of deacetylation. Excessive deacetylation can result in chitosan being difficult to hydrolyze by lysozymes [55][56].
2. Application of Chitosan and Derivatives in Transdermal Drug Delivery Systems
As mentioned above, chitosan and its derivatives have unique advantages in transdermal drug delivery systems. Some practical application forms of chitosan and its derivatives in transdermal drug delivery research (Figure 2) are summarized below.
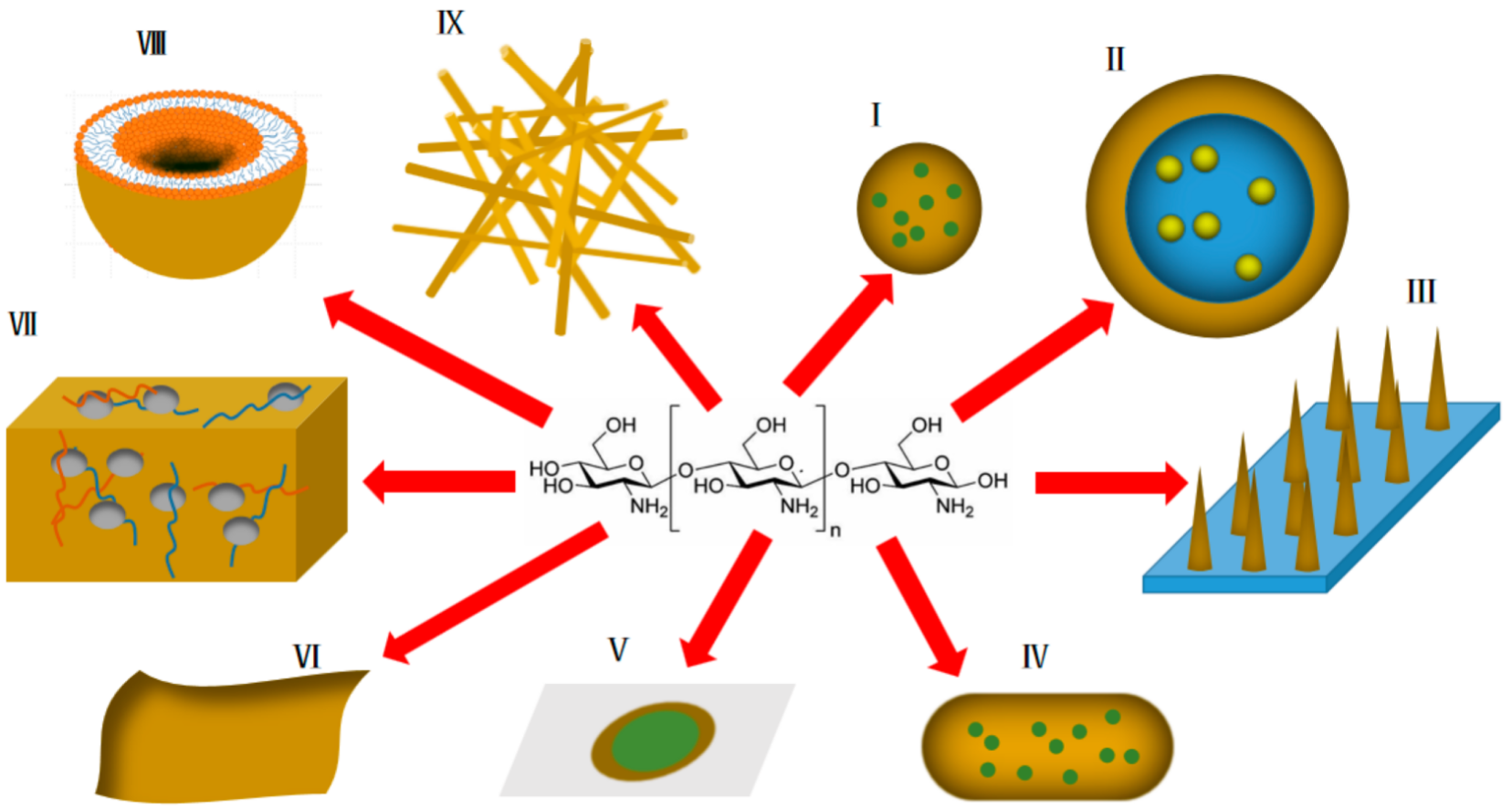
Figure 2. Various forms of application of chitosan and its derivatives in transdermal drug delivery systems (yellow represents chitosan and derivatives thereof). I: nanoparticles; II: emulsions; III: transdermal microneedles; IV: nanocapsules; V: transdermal patches; VI: transdermal membranes; VII: hydrogels; VIII: liposomes; IX: nano-scaffolds.
2.1. Nanoparticles
Nanoparticle refer to artificial solid particles with at least one dimension in the nanometer size range (0.1–100 nm) in three-dimensional space, also known as nano-dust or nano-powder. The ultrasmall size of nanoparticles enables them to have extraordinary permeability and great specific surface area, thus having great application value in transdermal drug delivery.
As the matrix of drug-loaded nanoparticles, chitosan has the advantages of sustained and controlled release, adhesion, permeability, and biocompatibility. Alaa Riezk et al. prepared amphotericin B-loaded chitosan nanoparticles for the treatment of cutaneous leishmaniasis [57]. Two amphotericin B chitosan nanoparticles with different electrical properties were prepared using positively charged sodium tripolyphosphate (TPP) and negatively charged dextran sulfate as crosslinkers. These two nanoparticles both showed high in vitro activity against Leishmania amastigotes. In addition, amphotericin B in aqueous solution could not penetrate the skin. When two types of AmB-loaded chitosan nanoparticles were applied to isolated mouse skin, AmB could slowly and limitedly penetrate the skin, and osmotic balance was achieved after about 20 h, indicating that chitosan nanoparticles could promote the in vitro skin permeation of amphotericin B. Delaying drug release is another effect of chitosan matrices containing nanoparticles. Salma et al. prepared tacrolimus-loaded chitosan nanoparticles using ion gelation technology for the treatment of psoriasis [58]. The in vitro skin permeation was determined over 24 h. The permeability of tacrolimus in the chitosan nanoparticles after 24 h was 24%; correspondingly, the permeability of tacrolimus cream after 24 h was 61%, i.e., the chitosan nanoparticles could significantly delay the release of tacrolimus and reduce the systemic toxicity of the drug. In addition, the 24 h skin deposition rate of tacrolimus cream was 11.4%, while that of the tacrolimus chitosan nanoparticles reached 75%, representing a benefit for the delivery of tacrolimus to the skin, which is the target site for the treatment of psoriasis. Psoriasis leads to skin sclerosis at the lesion site, greatly impeding the transdermal delivery of drugs. In this study, chitosan nanoparticles could improve the transdermal permeability of tacrolimus, delay its release from the cortex, prolong its action time at target sites, and reduce the effect of drugs on the whole body. Rada Al-Kassas et al. prepared a transdermal propranolol delivery system [59] with chitosan nanoparticles dispersed in gel, and they used porcine ear skin similar to human skin for the permeation study. The results showed that the system had thixotropy and slow-release effects, which were related to the local formation of a drug reservoir after chitosan nanoparticles were absorbed by skin. However, this conclusion requires further in vivo research support. Chitosan nanoparticles can neither increase nor reduce the toxicity of drugs toward skin keratinocytes and fibroblasts, which is a safe transdermal drug delivery system [60].
2.2. Emulsions
Emulsions are two-phase liquids that are immiscible with each other. One phase is dispersed as small droplets in the other phase to form a nonuniformly dispersed liquid formulation. Insoluble medicine is prepared into emulsions with large liquid drop dispersion, fast medicine absorption and drug effect exertion, and high bioavailability. Topical emulsions can improve permeability of the skin and mucosa, as well as reduce irritation. When in use, emulsions are uniformly coated on the medicinal part.
Chitosan mainly acts as a coating in transdermal milk. As a cationic polymer, chitosan and its derivatives can be coated on the outer layer of the emulsion to interact with the anionic surfactant in the emulsion through electrostatic interaction, thereby protecting the emulsion from agglomeration and maintaining its stability [61]. Taif Ali Khan et al. prepared a chitosan-coated 5-fluorouracil transdermal emulsion which showed good skin permeation characteristics compared with the 5- fluorouracil solution, which was related to the fluidization of the stratum corneum by chitosan and the surfactants in the emulsion, as described previously. Zeta potential is an important parameter representing the stability of an emulsion. A higher absolute value of the zeta potential indicates a more stable system. Zeta potential results showed that the chitosan-coated emulsion system had a higher positive zeta potential (+3.9 mV), i.e., the chitosan coating enhanced the stability of the emulsion system [62].
The mucosal adhesion of chitosan can prolong the retention time of an emulsion and facilitate continuous penetration. Babita Kumari et al. studied the effect of chitosan coating on the skin permeation properties of clotrimazole microemulsion [63]. The drug retention in rat skin after 8 h transdermal permeation was measured, and the results showed that the chitosan-coated clotrimazole microemulsion had higher drug retention in the skin compared with the control clotrimazole microemulsion (p < 0.05). Asma Sharkawy et al. used a chitosan/acacia clindamycin emulsion as the carrier to deliver trans-resveratrol, and the skin penetration test results showed that resveratrol had a high retention level in epidermis and dermis, which is of great significance for reducing the dosing time [64].
2.3. Transdermal Microneedles
Transdermal microneedles are microneedles that open the skin pipeline using micro- or nanotechnology, with the dual characteristics of injection administration and transdermal administration. When in use, as long as a small chip covered with microneedles is pasted on the skin, the microneedles can penetrate the cuticle layer of the epidermis, which functions as a barrier for drugs. A patch filled with drugs pasted on the skin allows their slow penetration into the epidermis, allowing them to be quantitatively and continuously administered. Compared with other transdermal preparations such as transdermal patches, transdermal membranes, creams, and the like, transdermal microneedles can directly penetrate the stratum corneum barrier to be administered to the dermis layer, have little extracutaneous residue, and can almost fully cross the stratum corneum, thereby greatly improving the skin permeation and absorption rate of the medicine [19][65]. Microneedles entering the skin can serve as a local drug reservoir to achieve continuous drug administration. Meanwhile, compared with traditional injection, transdermal microneedles avoid the pain caused by puncturing the skin when using a needle [66], which improves the tolerance of patients. In addition, since a large skin wound does not form, the risk of infection by injection is greatly reduced [67].
Water-soluble microneedles such as water-soluble polyvinylpyrrolidone (PVP), polyvinyl alcohol, and carboxymethyl cellulose (CMC) are rapidly dissolved after entering the skin, resulting in excessive drug release, while chitosan and its derivatives with relatively low water solubility can avoid this problem [34]. In addition, chitosan and its derivatives have good biocompatibility and biodegradability. Microneedles made of these materials do not need to be taken out after drug administration and can be degraded harmlessly in vivo. Zulcaif Ahmad et al. prepared a thiolated chitosan microneedle patch for transdermal delivery of tacrolimus, which achieved a high skin penetration efficiency (84%) and a longer release time (48 h 53.8 7.30%), with the sustained-release property related to the slow degradation of the needle after the microneedles entered the skin [68]. Mei-Chin Chen et al. developed a chitosan microneedle patch for the delivery of macromolecular drugs. The chitosan solid microneedles prepared by the double-casting method had sufficient mechanical strength to be inserted into pig skin at a depth of 250 μm in vitro and into rat skin at a depth of 200 μm in vivo. In vitro drug release studies showed the sustained release of BSA from chitosan microneedles with a cumulative release of approximately 95% observed over 8 days. This study confirmed that chitosan transdermal microneedles could achieve the skin delivery of macromolecules, which has significance for the development of transdermal delivery of biological macromolecules. On the basis of this study, the research group also investigated the possibility of chitosan microneedles as vaccine delivery vehicles. They developed a fully embedded chitosan transdermal microneedle. The delivery structure design enables the insertion array and the support array to be separated automatically after the microneedle enters the skin, thus realizing the sustained delivery of drugs for a long time without patches. Studies have shown that the microneedle system can prolong antigen exposure at the insertion site for up to 14 days and induce a robust immune response for at least 6 weeks [69].
Chitosan with natural anti-inflammatory and antibacterial properties can be used to promote skin wound healing. Junjie Chi et al. prepared a chitosan microneedle patch for promoting skin wound healing [70]. The wound models were treated with chitosan microneedle patches, microneedle-free patches, planar chitosan membranes, and PBS. The results showed that the expression levels of two proinflammatory factors (IL-6 and TNF-α) in the chitosan microneedle patch group were the lowest, indicating that, compared with other groups, the chitosan microneedle patch had the best effect on skin wound healing. Chitosan itself has a certain anti-inflammatory and antibacterial effect. The structural design of microneedles enhances the delivery efficiency of anti-inflammatory and antibacterial drugs, on one hand, and promotes the gas exchange between the outside world and regenerated tissues, as well as a reduction in the levels of inflammatory factors as compared with the planar chitosan membrane, on the other hand.
2.4. Nanocapsules
Drugs can be encapsulated in a capsule wall formed by inorganic or organic polymeric materials using microencapsulation technology to form a core–shell structure, which can isolate the drug active ingredient from the outside in the delivery process to avoid degradation and inactivation, with subsequent release upon reaching the target site. Microcapsules with a particle size of less than 1 μm are commonly referred to as nanocapsules. Nanocapsules not only have the effects of embedding, targeting, and sustained release, but can also improve the dispersibility, solubility, and stability of nanoparticles. In terms of transdermal drug delivery, nanocapsules can increase the permeability and adhesion of drugs to enhance the therapeutic effect.
María Javiera Alvarez-Figueroa et al. designed and prepared imiquimod-loaded chitosan nanocapsules for transdermal drug delivery, with an average particle size of 200 nm and a PDI less than 0.3, indicating a uniform system. The nanocapsules were stable under physiological conditions for at least 48 h, and the stability was good. The permeability of the nanocapsules was studied with pig skin. Raman spectrum analysis showed that the imiquimod band was observed at a depth greater than 20 μm, confirming that the nanocapsules could penetrate the stratum corneum and serve as an ideal platform for the transdermal delivery of imiquimod; furthermore, it was clear that chitosan played an important role in promoting permeability and isolating [71]. In addition, the mucoadhesive property of chitosan may also be responsible for the high permeability of the coated drug [72].
2.5. Transdermal Patches
Transdermal patches refer to thin-film preparations that can be pasted on the skin, where drugs enter the blood circulation through the skin. Transdermal patches consist of a backing layer, a drug reservoir, an adhesive layer, and a protective layer removed before use. The backing layer is located on the outermost side for isolating and protecting the drug delivery system from the outside world. The drug storage libraries are located in the middle. Some drug storage libraries also have controlled-release membranes to achieve sustained and controlled release of drugs. The adhesive layer is located on the innermost side and comes into direct contact with the skin for fixation. Compared with other transdermal preparations, the patch has the advantage that the drug can be stably retained on the skin for a long time without loss, ensuring the sustainability and stability of drug administration.
Chitosan and its derivatives are mostly used as dressing matrices in the dosage form of transdermal patches. Due to their good biocompatibility and biodegradability, chitosan transdermal patches are superior to other materials in promoting wound healing. The anti-inflammatory and antibacterial effects of chitosan can be synergistic with the delivered drugs. Specifically, Cs can accelerate the proliferation of fibroblasts and granulation, increase analgesic and hemostatic effects, stimulate neutrophils and IgM, interact with membrane phospholipid molecules, enhance the activation of macrophages and the production of extracellular matrix, and enhance antibacterial activity. Patches may also absorb excess inflammatory exudate to maintain a clean wound environment [73]. In addition, chitosan also has water retention property, which is of great significance for the water demand during the process of skin tissue repair and regeneration. Arash Ghalayani Esfahani et al. designed an electrophoretic deposition chitosan patch for local drug delivery. Using the water solvent, hydrogen was generated at the cathode electrochemical interface to form a porous structure. The use of the preparation to deliver the fat-soluble drug clobetasol propionate could achieve 80% drug release within 2 h, meeting the needs of local drug delivery [74]. In addition, chitosan could prolong the adhesion and release of the patch and reduce the drug administration time. Niranjan et al. prepared a PVA/curcumin/chitosan patch to improve the bioavailability of the water-insoluble drug curcumin [75]. The rat skin wound model was used to study the therapeutic performance of the patch. Compared to the corresponding commercially available creams, the PVA/curcumin/chitosan patch resulted in faster wound healing, manifested as uniform hair growth and reduced wound area in the dermal area. This was attributed to the fact that the sustained-release property of chitosan could reduce the number of patch replacements, which was conducive to accelerating wound healing.
This entry is adapted from the peer-reviewed paper 10.3390/ph15040459
This entry is offline, you can click here to edit this entry!
