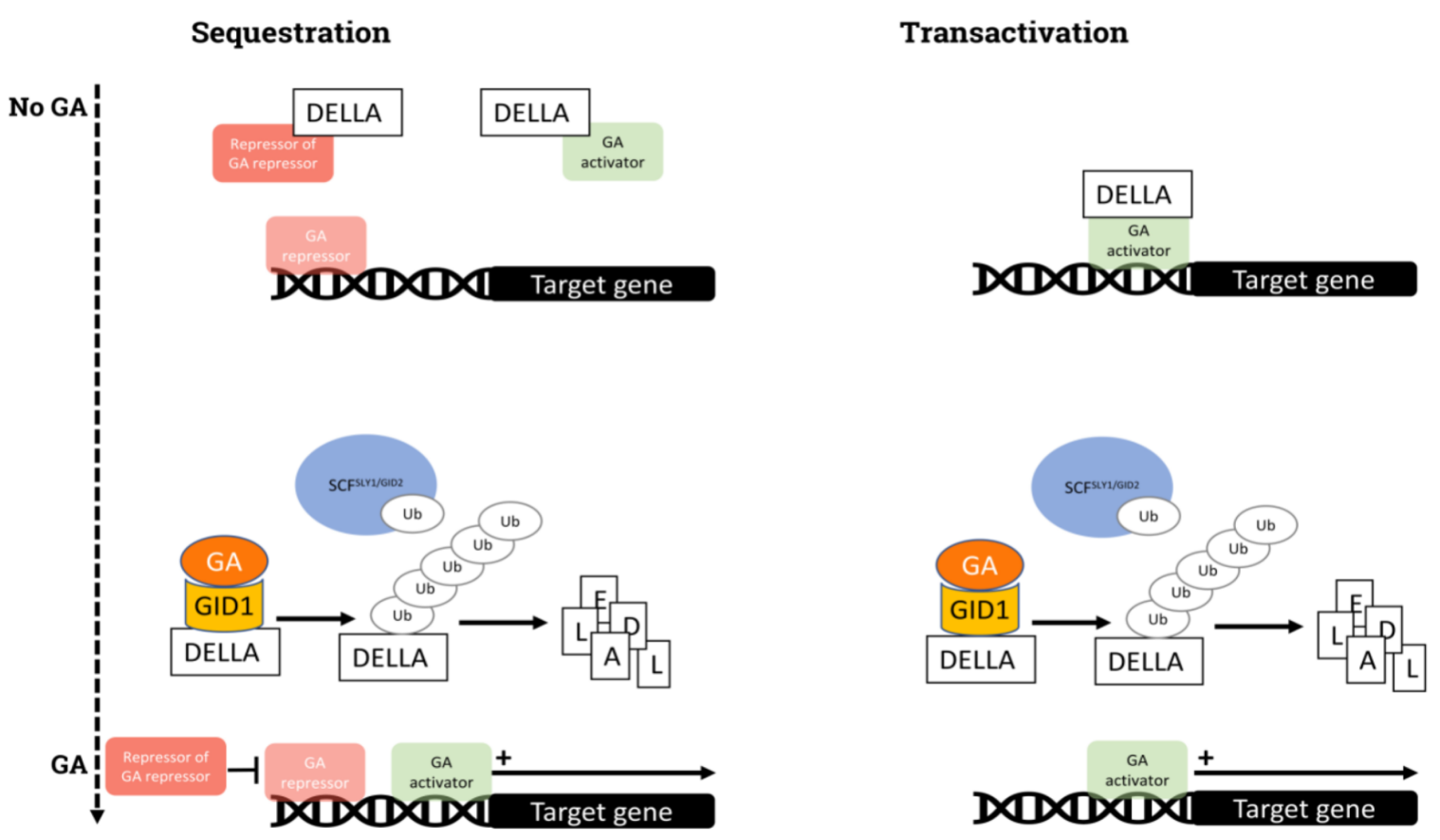
GA location and synthesis varies at the organ and tissue levels. GAs are maintained inside the cells by means of the ion trap mechanism
[22]. Weak acids such as GAs can enter the cytoplasm from the extracellular space due to their protonated state in that environment, and therefore have limited traveling ability on their own. Research has shown the relevance of GAs transport through plant tissues, but the involved mechanisms have not been characterized so far. No GA efflux proteins have been identified yet, although some proteins have been shown to import GAs into the cells. The abscisic acid (ABA) transporter ABA-IMPORTER TRANSPORTER 3 (AIT3) is able to import GA3, but it cannot import Jasmonic acid (JA) or Indole-3 acetic acid (IAA)
[23]. The Jasmonate-Isoleucine (JA-Ile) importer GLUCOSINOLATE TRANSPORTER 1 (GTR1) imports glucosinolates and GAs
[24]. The expression of the GAs importer protein, NITRATE TRANSPORTER 3/NITRATE PEPTIDE FAMILY (NPF3), is promoted by ABA and a lack of nitrogen, and is inhibited by GAs. Other NPF proteins are also able to import different GAs
[25]. Besides this, some data suggest that the sugar transporters BIDIRECTIONAL SUGAR TRANSPORTER SWEET (SWEET13/14) have GA transporter abilities
[26]. Due to the diversity of GAs in plants, it seems unlikely that a unique transport protein exists. Indeed, the current results suggest that bi- or multi-functional transporters are in charge of the transport across membranes.
The responses to GAs are influenced by several TFs, which modulate the activity of the DELLA proteins. SCARECROW-LIKE 3 (SCL3), a member of the GRAS family, competes with DELLA proteins for binding to INDETERMINATE DOMAIN (IDD) proteins, and modulates GAs levels through a feedback regulation between the DELLA/IDD and SCL3/IDD complexes
[46][47]. GAI-ASSOCIATED FACTOR (GAF1) is a TF that binds DELLA proteins, enabling the expression of the GA biosynthesis genes GA20ox2 and GA3ox1. However, when DELLAs are degraded in the presence of GAs, GAF1 binds to TOPLESS RELATED (TPR) and represses the expression of those same genes
[48]. This process depends on GA content, establishing a feedback regulatory mechanism
[49]. Nonetheless, other DELLA-independent homeostasis mechanism have been described. The zinc finger TF AXIAL REGULATOR YABBY1 (YAB1), which is expressed in response to GAs, binds to the GA-RESPONSIVE ELEMENT (GARE) domain in the promoter of the GA3ox2 gene, apparently blocking its expression and taking part in the negative feedback regulation of GA synthesis
[50]. In addition, OsYABBY4 interacts physically with the DELLA protein SLENDER RICE 1 (SLR1) and binds to the promoter region of GA20ox2, contributing to the modulation of GAs homeostasis
[51].
Two different N-acetyl glucosamination processes have been shown to influence GA-related responses. SECRET AGENT (SEC), a positive regulator of the GA response, induces a-GlcNAcylation (O-linked N-acetylglucosamine) of the RGA DELLA protein, preventing its union with several light- and hormone-related proteins and thus maintaining the effect of GAs
[52]. On the other hand, the interaction between SWITCH/SUCROSE NONFERMENTING (SWI/SNF), a chromatin-remodeling complex, SPINDLY (SPY), an O-GlNAc transferase, and DELLA negatively regulates GAs signaling
[53][54]. The SWIC3 core subunit of SWITCH/SUCROSE NONFERMENTIG has been shown to modulate GA responses by means of chromatin structure regulation
[55]. Indeed, epigenetic regulation has been shown to play a major role in GAs transcriptional activity. BRAHMA (BRM), a chromatin remodeling ATPase, induces the expression of genes related to GAs biosynthesis and signaling
[56], while PICKLE (PKL), another major chromatin remodeler, modulates the expressions of 80% of the GA-responsive genes
[57]. PKL seems to integrate outer and inner cues and hormone signaling pathways (such as GAs and Brassinosteroids (BRs)) to modulate gene expression
[58]. Therefore, the growth-promoting activity of GAs is subject to the control of many factors at different levels, ensuring that the plant invests energy in development only under the most favorable conditions.
3. Interactions with PGRs
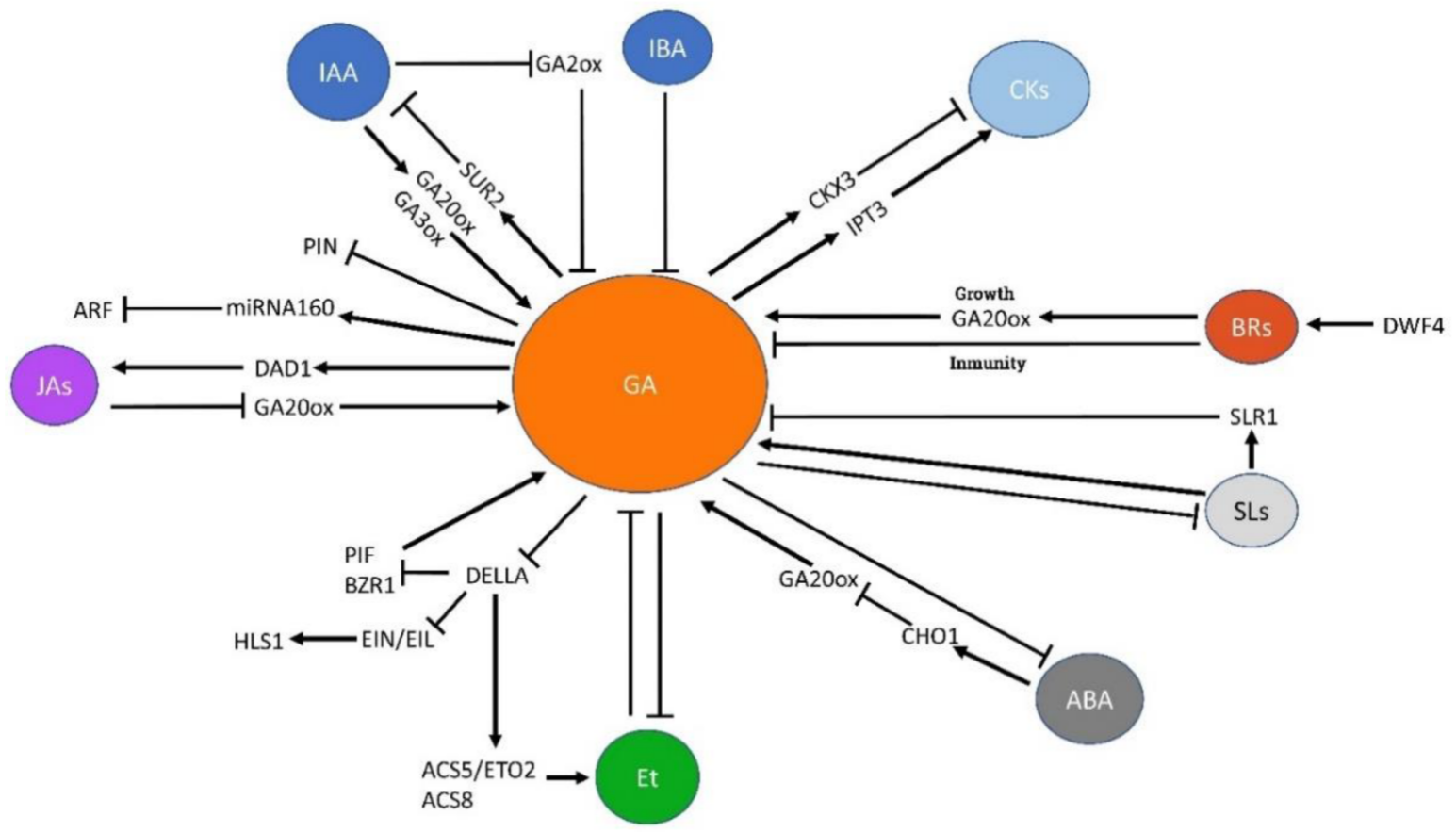
The relevance of the crosstalk between GAs and other hormones in the control of plant developmental processes has been reported in several species. The responses of tissues to PGRs depend on both the concentration of every growth regulator and the specific ratios between each set of hormones. This framework induces a highly complex scenario influenced by many factors, and particularly by genes involved in the homeostasis of every hormone (synthesis, transport…), which can be negatively or positively modulated by other hormones. Moreover, growth regulators can have synergistic or antagonistic effects on the activity of the same specific genes or proteins, thus adding extra levels of complexity to the processes under study. In the following section, researchers highlight what is known of the GAs’ interaction with other hormones (Figure 2) and with other potential plant growth regulators.
Figure 2. Schematic representation of the relations between GAs and other major PGRs. Arrows indicate activation and blunt-end lines indicate repression or inhibition. See text for details and references. ABA: abscisic acid, ACS5/ETO2: ACC synthase 5/ETO2, ACS8: ACC synthase 8, ARF: Auxin Response Factor, BRs: Brassinosteroids, BZR1: Brassinazole Resistant1, CHO1: Chotto1, CKs: Cytokinins, CKX3: Cytokinin dehydrogenase 3, DAD1: Defective anther dehiscence 1, DELLA: Della proteins, DWF4: Dwarf 4, EIN/EIL: ethylene insensitive 3/EIN3-like 1, Et: ethylene, GA: gibberellins, GA2ox: GA2-oxidase, GA3ox: GA3-oxidase, GA20ox: GA20-oxidase, HLS1: Hookless 1, IAA: indole-acetic acid, IBA: indole-butyric acid, IPT3: Isopentenyl transferase 3, JAs: Jasmonates, miRNA160: microRNA160, PIF: Phytochrome interacting factors, PIN: pin-formed, SLs: Strigolactones, SLR1: Slender Rice 1, SUR2: superroot 2.
3.1. Auxins
Auxins, mainly Indole Acetic acid (IAA), govern many aspects of plant growth and development, from the embryo stage to senescence. IAA promotes GA synthesis by activating GA3ox and GA20ox and deactivating GA2ox, as seen in Arabidopsis, rice and pea
[59][60][61]. The positive influence of auxins on GA content seems to occur in tissue-specific responses, such as those taking place in the roots of pea plants
[62] or during fruit set in tomato
[63]. On the other hand, GAs modulate auxin-related genes, although the outcomes of these responses depend on the specific set of GA-induced Auxin Response Factors (ARFs). Whilst they promote hypocotyl elongation via ARF6 and ARF8
[64], during grape parthenocarpy ARF10/16/17 are negative regulated by GA3 through the activity of the microRNAs miR160a/b/c
[65].
Furthermore, GAs modify the expressions of several auxin transporters
[66]. GA biosynthesis and signaling-deficient mutants in Arabidopsis show a reduced activity of PIN-FORMED (PIN) protein auxin transporters (PIN1, PIN2, PIN3), indicating that GAs are needed for the proper function of PIN proteins, as the wild-type phenotype is restored upon GA treatment
[67]. This GA-dependent regulation has biological effects, such as gravitropism modulation in Arabidopsis roots via PIN2 stabilization
[67][68] or xylogenesis promotion in
Populus by means of PIN1 upregulation
[69]. In
Eucalyptus roots and stems, exogenous GA3 treatment promotes xylogenesis and alters the expression of genes not only related to GA biosynthesis, but also to auxin and secondary cell wall formation
[70]. Xylem differentiation from cambium cells was also reported in hypocotyl cuttings of
Pinus radiata seedlings treated with indole-butyric acid (IBA) and GA3
[71].
To further complicate their interplay, auxin content and signaling are also influenced by GAs. In
Eucalyptus roots, GAs upregulate SUPERROOT2 (SUR2), which is involved in auxin homeostasis
[70], while in Arabidopsis GA3 improves root responses to exogenous IAA through the modulation of auxin transporters (AUX1 and PIN1, PIN2 and PIN3) and signaling, as these effects are not detected in Arabidopsis signaling mutants (
tir1-1 and
axr1-3)
[72].
The interaction between auxins and GAs occurs at many levels, including signaling, metabolism or gene expression, and in many cases in a tissue-specific manner. Although a clear relationship between both hormones cannot be stated, as they share a positive influence on some aspects of development, they seem to present a synergistic character.
3.2. Brassinosteroids
BRs and GAs appear to play a cooperative role in their physiological effects, as well as in GA biosynthesis regulation. The TF BRASSINAZOLE RESISTANT 1 (BZR1) controls BRs responses in plants, and is activated by GAs after DELLA degradation. Similarly, BRs take part in plant responses to light by enhancing the transcriptional activity of PHYTOCHROME INTERACTING FACTORS (PIF) TFs
[64][73]. This is a potential intersection between BRs and GAs, since GAs allow the activity of PIF4
[74] and, conversely, PIF4 modulates the expression of
GA3ox and
GA20ox [75]. The over-expression of the BRs biosynthesis gene DWARF 4 (
DWF4) and of
GA20ox leads to an increment in the GA levels
[76], as well as in the expression of
BZR1 and
BRI1 EMS SUPPRESSOR 1 (BES1)
[77]. BRs cooperate with GAs to modulate plant height in rice through the enhancement of GA synthesis
[78]. At physiological levels, BRs collaborate with GAs to promote cell elongation, but at high BRs levels GA biosynthesis is inhibited in rice
[79]. Moreover, the inhibitory effect of high BRs levels on GAs biosynthesis is a strategy of some oomycetes to suppress immune responses in rice
[80]. Therefore, there seems to be a molecular mechanism by which BRs and GAs exert mutual control over each other’s activity. However, in sunflower, Arabidopsis
[81] and pea
[82], this BRs-GAs interaction has not been found, suggesting the species- or stage-specific conditioning of these relations.
3.3. Ethylene
The gaseous hormone ethylene and GAs act antagonistically on root growth, as primary root growth is enhanced by GAs and repressed by ethylene. This is an example of their contrary effects on development. This opposite behavior might rely on the ability of ethylene to modulate GAs homeostasis. Several studies have shown the capacity of ethylene to negatively modulate or alter GAs biosynthesis genes in Arabidopsis
[83][84]. Moreover, it has been suggested that ethylene regulates both biosynthetic and catabolic GA genes in this species
[85]. This type of modulation has also been found in tomato and pea
[86][87].
However, in Arabidopsis, GAs–ethylene interaction seems to be crucial for the development of the apical hook. After DELLA degradation, the activation of ETHYLENE INSENSITIVE 3/EIN3-LIKE 1 (EIN3/EIL1) by GAs promotes
HOOKLESS1 (HLS1) expression. HSL1 modulates apical hook formation and prevents its premature opening in etiolated seedlings, showing once again the close interaction between light and GAs
[88][89]. Besides this, GAs enhance the correct development of the apical hook by modulating
PIN3 and
PIN7, and promoting ethylene biosynthesis by activating
ACC SYNTHASE5/ETO2 (ACS5/ETO2) and
ACC SYNTHASE 8 (ACS8) [90]. The interaction of DELLA proteins GIBBERELLIC ACID INSENSITIVE (GAI), RGA and RGA52 with the ethylene response factors RELATED TO APETALA (RAP) RAP2.3 and RAP2.12 blocks their transcriptional activity, thus repressing their own action and enhancing the apical hook opening
[91]. Overall, there seems to be a complex relation between GAs and ethylene, which can have contrasting characteristics according to the specific process under study.
3.4. Abscisic Acid
ABA and GAs have a reciprocal antagonist effect in biosynthesis modulation. ABA and GAs balance is critical in different biological processes, but it is particularly relevant for seed dormancy and germination. ABA-INSENSITIVE4 (ABI4) promotes ABA synthesis and
GA2ox7 expression, inhibiting seed dormancy
[92][93]. In addition, ABA, by means of
CHOTTO1 (CHO1), inhibits
GA20ox2 and reduces GA levels during seed germination. An inhibitory effect of GA on ABA synthesis has also been reported
[94]. Several external cues, such as temperature, water stress and light, are also integrated within the ABA/GA balance to ensure the fine-tuning of seed germination.
3.5. Other PGRs
Jasmonic Acid (JA) plays important roles in several biological processes, but particularly in plant defense and responses to environmental conditions. Due to the different processes GAs and JA govern, they usually show an antagonistic relationship mediated by JASMONATE-ZIM DOMAIN (JAZ) and SLR1
[95]. DELLA proteins interact with the JA signaling machinery, enabling the enhanced activity of one hormone or the other, although some synergistic effects have also been found (reviewed in
[96]). In tobacco, Jasmonates reduce
GA20ox2 levels, thus lowering GA content
[97].
DEFECTIVE ANTHER DEHISCENCE 1 (DAD1), which intervenes in jasmonate metabolism, is upregulated by GAs in
Eucalyptus [70]. Interestingly, the interaction of JA/GA seems to present a cooperative nature in the formation of flower tissues, at least in model species such as Arabidopsis and rice (Castro-Camba et al., under review).
The putative interactions between cytokinins and GAs are not clearly defined, mainly due to the lack of precise data, although they seem to act antagonistically in several processes such as phase change
[98]. GAs activate different cytokinin catabolic genes in Medicago truncatula
[99] and tall fescue
[100], resulting in the inhibition of tillering in the latter. On the other hand, the activation of cytokinin receptor CYTOKININ RESPONSE 1 (CRE1) by cytokinins reduces GA levels in
M. truncatula [101], whereas SPY, which represses GAs signaling, acts as a positive regulator of cytokinins signaling
[102]. However, in
Eucalyptus, GAs have been shown to activate ISOPENTENYL TRANSFERASE 3 (IPT3), which intervenes in the metabolism of cytokinin,
[70]. Moreover, CKs and GAs act synergistically in the regulation of morphological and physiological traits in
Polygonum cuspidatum in response to nitrogen availability, with GAs also integrating light cues. Under high nitrogen conditions, the levels of endogenous CKs and GAs are increased, indicating that both hormones are involved in biomass allocation in response to nitrogen availability
[103].
Strigolactones (SLs) were first identified as molecules synthesized by parasitic plants, but were later recognized as endogenous phytohormones. SLs and GAs have similar perception and signaling mechanisms
[104][105]. Shoot elongation and tiller bud outgrowth seem to be under the control of the crosstalk between SLs and GAs
[106]. However, the outcome of this crosstalk might depend on the process under analysis. SLs are required for the interaction between DWARF 14 (D14) and SLR1 proteins, negatively regulating GA signaling
[107]. On the other hand, GA signaling represses SLs biosynthesis genes
[108]. Bud outgrowth in
Jatropha curcas is promoted by GAs but inhibited by SLs
[109]. However, SLs modulate ABA/GA ratio, lowering ABA synthesis and increasing GA accumulation during the germination of thermo-inhibited seeds
[110], while SLs regulate shoot elongation in rice, influencing GA metabolism and signaling
[111].