Myrtus communis subsp. communis is an evergreen shrub or a small tree, growing spontaneously throughout the Mediterranean basin. The stem is branched from the basal portion and the bark is brownish or reddish in color. The leaves are simple, opposite, sessile or sub-sessile, glossy, and dark green in color, lanceolate or ovoid-elliptical in shape with entire or slightly revolute margins and acute apices; they are very aromatic due to the presence of numerous secretory cavities. The flowers, white in color with yellowish streaks, are solitary or coupled at the leaf axil. The fruits are ellipsoidal or subspherical berries, red-violet or blackish in color at maturity, with persistent calyx residues.
- myrtle
- botanic gardens
- secretory cavities
- essential oils
- α-pinene
- 1
- 8-cineole and linalool chemotype
- light microscopy
- GC-MS
- Open science
1. Introduction
Myrtle has a consolidated ethnobotanical tradition and is used in different parts of the world, against colds and coughs [1], for self-medication of digestive problems, for skin disorders [2], for the treatment of obesity, hypercholesterolemia, and diabetes [3][4]. The essential oils (EOs), obtained from shoots, leaves and sometimes flowers and berries, are used eminently in perfumery. The berries are also employed in the production of bitters and famous liquors. Due to its broad use in folk medicine, myrtle has been widely investigated, especially with regards to the EO composition [5][6][7][8][9][10][11]. In Italy, previous studies were focused on plants from Sardinia, Sicily, Campania, and Liguria regions [8][9][12][13][14][15]. Concerning literature data on the micromorphology, previous investigations were focused on the structure and ontogeny of the secretory cavities of leaves and flowers by means of both light and electronic microscopy [16][17][18][19].
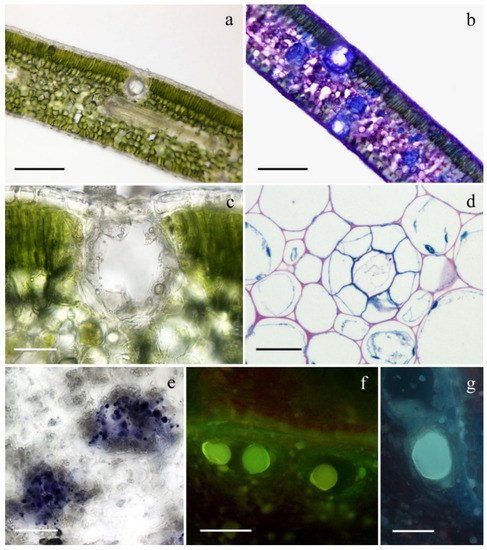
2. Micromorphological Investigation
3. Phytochemical Investigation
In 2018 the leaves were treated according to four different preparation methods and separately hydro-distilled: as fresh material (FL), after freezing at −20 °C (20FL), after freezing at −80 °C (80FL), and after air-drying at room temperature (DL). The profiles of the leaf EOs are reported in Table 1.
| LRI | Class | Compound | Relative Abundance (%) | ||||
|---|---|---|---|---|---|---|---|
| FL | 80FL | 20FL | DL | ||||
| 1 | 769 | OTHER | hexanal | 0.1 | tr | 0.1 | tr |
| 2 | 844 | OTHER | 2-hexenal | 0.6 | 0.3 | 1.2 | 0.6 |
| 3 | 912 | OTHER | isobutyric acid, isobutyl ester | 1.0 | 0.4 | 0.9 | 1.1 |
| 4 | 927 | MH | α-thujene | 0.1 | 0.2 | 0.4 | 0.6 |
| 5 | 935 | MH | α-pinene | 38.6 | 36.2 | 41.2 | 41.6 |
| 6 | 961 | MH | camphene | 0.2 | 0.1 | 0.1 | 0.1 |
| 7 | 983 | MH | β-pinene | 0.3 | 0.1 | 0.2 | 0.3 |
| 8 | 990 | MH | myrcene | 0.6 | 0.2 | 0.3 | 0.3 |
| 9 | 1005 | MH | α-phellandrene | 0.2 | 0.2 | 0.1 | tr |
| 10 | 1008 | MH | 3-carene | 0.8 | 0.2 | 0.3 | 0.5 |
| 11 | 1019 | MH | α-terpinene | 0.1 | 0.1 | 0.1 | 0.1 |
| 12 | 1028 | AH | o-cymene | 0.1 | - | 0.3 | 0.5 |
| 13 | 1030 | MH | limonene | - | - | 2.9 | 3.3 |
| 14 | 1036 | MH | cis-sabinene hydrate | 0.7 | - | - | - |
| 15 | 1049 | OM | 1,8-cineole | 31.0 | 25.5 | 28.2 | 26.1 |
| 16 | 1052 | MH | α-ocimene | 0.4 | 0.3 | 0.4 | 0.3 |
| 17 | 1064 | MH | γ-terpinene | 0.5 | 0.3 | 0.5 | 0.4 |
| 18 | 1074 | OM | cis-linalool oxide | 0.1 | tr | 0.2 | 0.3 |
| 19 | 1088 | MH | α-terpinolene | 0.5 | 0.5 | 0.7 | 0.7 |
| 20 | 1100 | OM | linalool | 10.7 | 28.6 | 18.7 | 18.2 |
| 21 | 1125 | OM | fenchol | 0.1 | tr | tr | - |
| 22 | 1140 | OM | pinocarveol | 0.2 | tr | 0.2 | - |
| 23 | 1143 | OM | nerol oxide | 0.1 | - | - | - |
| 24 | 1154 | OM | pinocarvone | 0.1 | - | - | - |
| 25 | 1159 | OM | δ-terpineol | 0.1 | tr | - | - |
| 26 | 1162 | OM | borneol | 0.1 | tr | - | - |
| 27 | 1168 | OM | terpinen-4-ol | 0.5 | 0.2 | 0.2 | 0.3 |
| 28 | 1202 | OM | α-terpineol | 1.8 | 2.1 | 1.4 | 2.0 |
| 29 | 1248 | OM | linalyl acetate | 0.9 | 0.6 | 0.4 | 0.8 |
| 30 | 1255 | OM | trans-geraniol | 1.3 | 0.2 | - | - |
| 31 | 1296 | OM | trans-pinocarvyl acetate | 0.1 | tr | - | - |
| 32 | 1318 | OM | methyl geranate | 0.1 | tr | - | - |
| 33 | 1347 | OM | α-terpinyl acetate | 0.7 | 0.3 | - | - |
| 34 | 1356 | OM | nerol acetate | 0.5 | 0.2 | - | - |
| 35 | 1376 | OM | geranyl acetate | 0.8 | 0.8 | - | - |
| 36 | 1402 | OM | methyleugenol | 0.9 | 0.3 | - | - |
| 37 | 1423 | SH | β-caryophyllene | 1.0 | 0.4 | 0.5 | 0.6 |
| 38 | 1440 | SH | aromadendrene | 0.1 | - | - | - |
| 39 | 1461 | SH | humulene | 1.0 | 0.5 | 0.4 | 0.6 |
| 40 | 1492 | OTHER | 2-tridecanone | 0.1 | - | - | - |
| 41 | 1518 | OTHER | durohydroquinone | 0.8 | 0.7 | - | 0.7 |
| 42 | 1562 | OS | trans-nerolidol | 0.4 | - | - | - |
| 43 | 1588 | OS | caryophyllene oxide | 0.4 | tr | - | - |
| 44 | 1603 | OS | trans-bisabolene oxide | 0.1 | tr | - | - |
| 45 | 1616 | OS | humulene oxide II | 0.3 | tr | - | - |
| 46 | 1660 | OTHER | 5,8,11-heptadecatrien-1-ol | 0.1 | - | - | - |
| 47 | 1705 | OTHER | methyl ketostearate | tr | 0.1 | - | - |
| Oil yields | 0.36% | 0.46% | 0.49% | 1.08% | |||
| Total identified | 98.9 | 99.9 | 99.7 | 100.0 | |||
| Monoterpene hydrocarbons (MH) | 42.9 | 38.5 | 47.2 | 48.2 | |||
| Oxygenated monoterpenes (OM) | 50.0 | 59.0 | 49.2 | 47.7 | |||
| Sesquiterpene hydrocarbons (SH) | 2.1 | 0.9 | 0.9 | 1.3 | |||
| Oxygenated sesquiterpenes (OS) | 1.1 | 0.1 | tr | tr | |||
| Aromatic hydrocarbons (AH) | 0.1 | tr | 0.3 | 0.5 | |||
| Other compounds (OTHER) | 2.7 | 1.5 | 2.1 | 2.4 | |||
This entry is adapted from the peer-reviewed paper 10.3390/plants11060754
