Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell & Tissue Engineering
Recently, targeted modulation of the immune response via biotechnological approaches and biomaterials has gained attention as a means to restore the pro-healing phenotype and promote tissue regeneration. In order to fully realize the potential of these approaches in traumatic wounds, a critical and nuanced understanding of the relationships between immune dysregulation and healing outcomes is needed.
- injury
- wounds
- trauma
- inflammation
- bioengineering
- immunoengineering
1. Cutaneous Wound Healing
Wounds are often defined as peripheral or extensive (deeper) disruptions of the epithelial integrity of the skin, which extends to subcutaneous tissue with damage to underlying structures such as tendons, muscles, vessels, nerves and parenchymal organs [10]. Functional wound healing is a well-orchestrated sequence of overlapping phases, such as coagulation, inflammation, proliferation and remodeling. Figure 1 illustrates the sequence of wound healing events following an acute injury.
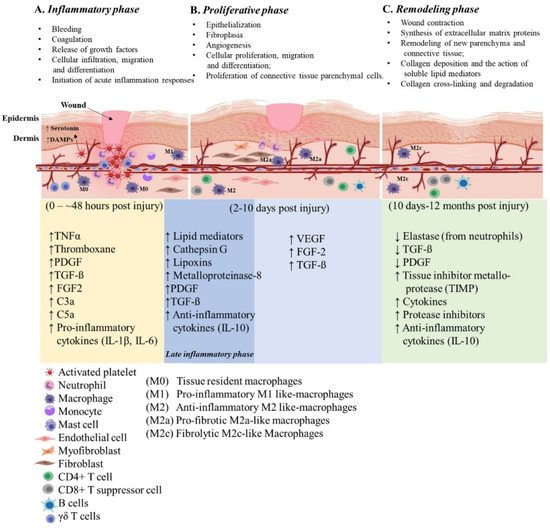
Figure 1. Illustration of the cutaneous wound healing process. The three phases of open wound healing are inflammatory phase: 0–~48 h (yellow area); proliferative phase: 2–10 days (blue area); and remodeling phase: 10 days–12 months (green area). Overlap of the inflammatory and proliferative phases (dark blue area) is also referred to the late inflammatory phase. The time scale starts at the time of injury and extends through 12 months post injury. The upward arrows indicate increased expression and the downward arrows indicate decreased expression of the molecules. This figure was created with BioRender.com (accessed in June 2020).
2. Immune Engineering Approaches to Modulate Inflammation
Immunoengineering represents the discipline of bioscience and technology that enhances antigen presentation, revive innate immunity, delivery of active therapeutic to specifically modulate immune cells, ranging from synthetic drug to biologics-including cells and cellular factors, and implementing engineered biocompatible materials to unravel the immune system function and regulation in health and disease [212,213]. Recent advance in immune biology, analytical and engineering tools has allowed deconvolution of complex immune function discerning to a variety of diseases, resulting in the development of novel therapies to specifically modulate and control dysregulated immune functions and restore normal physiological state. Several therapeutic options have been researched, including, cellular engineering, cytokine therapy, nucleic acid-based immunotherapy, synthetic drug delivery, and bio-engineered material based approaches (Figure 2). The following sections shed light on the recent advancements on the current and emerging immune engineering approaches developed to target acute immune responses following a traumatic insult to the skin and the underlying muscle. Additionally, a summary of select immune engineering approaches and their applications can be found in Table 3.
Table 3. Summary of selected immune engineering approaches to promote wound healing.
| Approach | Injury Type | Outcomes | Ref. |
|---|---|---|---|
| Nucleic acid and aptamers based immune targeting | |||
| Intra-arterial VEGF gene delivery by magnetic DNA nano spheres | Rabbit limb ischemia model |
|
[214] |
| Nanoparticle-based pcDNA3.1-CYP2J2 plasmid DNA (pDNA) delivery system (nanoparticle/pDNA complex) | Mouse limb ischemia model |
|
[215] |
| Hydrogel loading plasmid DNA encoding VEGF | Mouse burn wound model |
|
[216] |
| Theranostics immune targeting | |||
| Smart flexible electronics-integrated wound dressing | Pig full thickness wound model |
|
[217] |
2.1. Nucleic Acid and Aptamers Based Immune Targeting
The advent of DNA nanotechnology has paved the way for exploration of its biological application, including tissue regeneration, cancer therapy, inflammatory diseases, imaging, diagnosis, drug delivery and therapeutics [218]. Due to non-immunogenic deoxyribonucleic basic component, DNA nanoparticles present low immunogenicity and their internalization would not intensify immunoreaction. DNA nano particle have been widely applied in tissue regeneration and immune stimulation. DNA-encoding VEGF were designed to target inflammation in both chronic and acute wounds. A gene-activated bilayer dermal equivalents (Ga-BDEs) developed by loading the nano-sized complexes of Lipofectamine 2000/plasmid DNA-encoding VEGF into a collagen-chitosan scaffold/silicone membrane bilayer dermal equivalent was shown to have a dual functions of immunomodulation and pro-angiogenesis simultaneously [219]. Magnetic DNA nanospheres containing expression plasmids DNA (pDNA) encoding VEGF was able to promote angiogenesis in ischemic limb by alleviating the high oxidative stress and inflammatory micro-environment in both mouse and rabbit models [214,215]. In another study, pDNA-VEGF accelerated excisional burn wound healing, by inhibiting inflammation response; IL-1β or TNFα expression were significantly reduced, thus promoting microvascular formation [216]. The advantage of DNA nanoparticle is it can be conveniently designed to desired shape, to include single layer, wired frame and multilayer structures. This versatility has led to the design of advanced DNA nano materials such as multifunctional and intelligent DNA nano device, nano flowers (NFs), nano circuits and nano robots capable of targeting and delivering payloads such as drugs, fluorescent optical labels and even aptamers [220,221,222,223,224].
Single stranded DNA (ssDNA) or aptamers, are a special class of nucleic acid molecules can form secondary and tertiary structures capable of specifically binding proteins or other cellular targets [225,226]. Aptamers are selected by a process called systematic evolution of ligands by exponential enrichment (SELEX), in which DNA or RNA molecules are selected by their ability to bind their targets with high affinity and specificity, comparable to those of antibodies [227,228]. Harnessing the selective binding ability with specific target at high precision, aptamers are designed to interact with complementary molecules targeting the immune system. Following an acute injury, exposure of wound surface to any bacterial pathogens activates antigen-specific acquired immunity signaling pathways such as MAPK and nuclear factor-kB (NF-kB) via the activation of Toll-like receptors (TLRs) on neutrophils, monocytes, macrophages as well as B cells and T cells [229] favoring the release of inflammatory cytokines. There are aptamers specifically that bind to TLRs (2, 9) and cytokines such as IL-6 receptor (IL-6R), IL-10R to dampen the severity of cytokine storms [230,231]. Thus, far most of the aptamers are designed to address pathological conditions such as cancer, atherosclerosis, immunodeficiency and autoimmunity [232,233,234,235]. In the arena of wounds and traumatic injuries, aptamers are used as a potential molecular probes to examine bacterial infection, thus allowing the remote detection of a pathogens [236,237]. Recently, a much more advanced point-of-care in situ platform is developed to monitor wound status beyond detection of pathogenic bacteria. This device comprises flexible multiplexed immunosensors integrated with aptamers sensor array for measuring inflammatory mediators such a TNFα, IL-6, IL-8 and TGFβ1, and Staphylococcus aureus. Additionally, the array can monitor vital parameters at the wound site such as temperature and pH. The entire immune-platform is a microfluidic device capable of monitoring each of the aforementioned analyte through wound exudate sample collector. More importantly the entire platform is built on flexible electronics for wireless, smartphone-based data readouts [238].
2.2. Theranostics Immune Targeting
A recent symposium conducted by the National Academy of Engineering on leading-edge engineering technology identified immune theranostics as one the promising and innovative technological advancements to harness the full potential of immunotherapy in the treatment of a wide range of inflammatory disorders [239]. Theranostics is an engineering approach that combines delivery of therapeutic and diagnostic agents. Theranostic immunotherapy focuses on development of nanoscale biomaterials (NBMs) to modulate the immune system [240,241]. Conceptually, theranostic NBMs are custom targeting nano-sized materials tethered with antibodies, peptides, aptamers and other molecular recognition motifs [242]. The current theranostic NBMs are primarily used to target inflammation-driven pathologies. These immune NBMs specifically directs the immune balance toward either a pro- or anti-inflammatory state depending on the desired outcome for a given disease [243]. Theranostic hydrogel approach to improve acute wounds has been recently attempted. A sophisticated hydrogel from chemically modified hyaluronic acid (HA), dextran (Dex) and β-cyclodextrin (β-CD) was designed to deliver VEGF plasmid as the anti-inflammatory and pro-angiogenic components. The hydrogel accelerated the splinted excisional burn wound healing by inhibiting inflammation response and the pDNA-VEGF promoted new patent blood vessel formation characterized by co-localized positively stained CD31 and α-SMA cells within the wound bed [216]. While the theranostic approach to treat acute injury is still in infancy, still underlying mechanistic of an NBM-ligand-mediated inflammatory targets could be applied to design novel immune-theranostics to regulate inflammatory status of an acute injury.
The rising need of personalized medicine to deliver on-demand drugs has led to development of strategies to engineer materials that will respond to local changes in the wound and release therapeutics. In a recent study, a smart flexible electronics-integrated hybrid wound dressing was developed integrating a polydimethylsiloxane-encapsulated flexible electronics with a temperature sensor and ultraviolet (UV) light-emitting diodes, and polyethylene glycol diacrylate (PEGDA) hydrogel loaded with gentamycin responsive to UV activation to treated infected 3 cm diameter full thickness model. The developed smart dressing was capable of monitoring temperature and using a NIR sensor detects infection induced hyperthermia. Subsequently, the integrated LED triggers antibiotic release upon UV activation [217]. In another recent research study, a smart wound dressing features glowing nano sensors with fluorescent magnesium hydroxide nano sheets (Mg(OH)2-NS) was developed. Magnesium’s antimicrobial, anti-inflammatory and biocompatible properties are well known. The developed smart dressing with capability to mitigate medically relevant bacterial and fungal infections were doped with pH probe to monitor wound status in real time [244].
The next generation of theranostics will include a data-driven wound healing assessment and management system by leveraging machine-learning and deep-learning frameworks. Integrating immune engineering, smart materials, bioelectric technologies will enable monitoring of temperature, moisture, pressure, pH and cytokine, and will also enable precise, on-demand release of therapeutic for better wound management and healing.
3. FDA Position Statement
For successful clinical translation, one should adopt standard operating procedures designed to generate immunotherapeutics under current good manufacturing practice regulations. The FDA’s Center for Devices and Radiological Health (CDRH) classified wound dressings combined with drugs as “wound dressings containing drugs”, under product code “FRO.” These products include solid wound dressings, gels, creams, ointments and liquid wound washes. Within this classification, wound dressings combined with a drug are generally regulated as combination products. By definition, a combination product is comprised of two or more constituent parts (i.e., drug/device, biologic/device, drug/biologic or drug/device/biologic) and should meet the requirements of a combination product under FDA 21 Code of Federal Regulation (CFR) 3.2. There are several COX-2 selective NSAIDs, and prescription or over-the-counter (OTC) non-selective NSAIDs for use to treat a variety of general ailments. Some of the examples of COX-2 selective agents include Celecoxib, Valdecoxib and Rofecoxib. However, after approval, the FDA made strongly worded black-box warnings for each of the three COX-2 inhibitors currently approved in the United States [245]. A quest to identify specific approved anti-inflammatory drug and drug eluting dressing followed, and in 2016, under 21 CFR 3.2, the FDA recommended ingredients that are contained within unclassified and cleared wound dressings. Within the comprehensive list of drugs, both inorganic and organic chemical constituents are included. The wound dressing Fortaderm®, for example, is a collagen-based polyhexamethylene biguanide (PHMB) antimicrobial wound dressing that gained FDA approval in 2001. Other chemical drugs listed are magnesium-based inorganic compounds, for example, magnesium oxide and sulfate. Owing to the antimicrobial efficiency of magnesium, magnesium hydroxide-based compounds and magnesium doped dressings are considered for smart dressings with the capability to mitigate medically relevant bacterial and fungal infections [244].
The FDA has a Center for Biological Evaluation and Research (CBER), which regulates human cells, tissues and cellular and tissue-based products (HCT/P). FDA code and the definition of the use of cells as therapeutics under biologics are complex. Our recent review provides a succinct mechanism of FDA approval mechanism of drugs, biologics and medical devices for wound healing purposes [246]. Wherein, drugs and biologics (i.e., stem cell therapies) take the longest time to receive an FDA approval, requiring both pre-clinical animal studies followed by three phases of clinical trials. While there are a number of clinical trials initiated to treat acute wounds with the primary aim of skin regeneration and closure, curb-siding inflammation has been a secondary point of determination [246]. Still, there is a long way to go for an exclusive cell therapy specifically addressing modulation of the inflammatory response of an acute injury. To date, stem cell-based therapies are the most likely candidates to show promising result in clinical trials.
The FDA position also stands true for cell secretomes, due to hurdles in clinical translation of the complex EV populations. A true success of EV research relies on manufacturing and successful application to treat human etiologies, while being in compliance with existing regulatory frameworks. Strategies for a methodical, safe and efficacious EV scale-up manufacturing and pharmaceutical use have been laid out as positional statements by the members of the International Society for Extracellular Vesicles (ISEV) and of the European Cooperation in Science and Technology (COST) program of the European Union. As the classification defines “subsequent requirements for manufacturing, quality control and clinical investigation”, it is of major importance to define if EVs are considered the active drug components or they primarily serve as drug delivery vehicles. Taking this into consideration, this important FDA guideline requires that a product should be manufactured under the current good tissue practice (CGTP) by establishments that perform a manufacturing step under contract, agreement or other arrangement for another HCT/P establishment. The core CGTP requirements include: facilities, environmental control, equipment, supplies and reagents, recovery, processing and process controls, storage, receipts, pre-distribution shipment and distribution of HCT/P, and donor eligibility determinations, screening and testing. To qualify for clinical usage, EVs typically follow a sequential step, which are, EV-based investigation medical product (IMP)/Investigation new drug (IND) filing, potency evaluation in vitro and in vivo according to the CFR of the FDA, route of administration (systemic versus local application), single versus multiple administration and dosage of the IMP/IND per treatment, identification of personalized versus common (off-the-shelf) use, source cells and their current good manufacturing practice (GMP) production documentation, naturally or endogenously loaded versus artificially or externally loaded EVs. Normally, EV purification is performed using tangential flow filtration (TFF) combined with a short ultracentrifugation (UC) step [247,248]. A thorough quality control (QC) and documentation of the final product testing, including characterization data, and batch-to batch consistency records are pre-requisites to achieve the approval for clinical testing [249,250]. In addition, the GMP manufacturing method assures high exosome yield (>1013 particles) and consistent removal (≥97%) of contaminating proteins [251]. To date, EVs are under clinical investigation, and a comprehensive list of on-going trials are published recently in the position statement article published by EVOLVE France, (Extracellular Vesicle translatiOn to clinicaL perspectiVEs), created in 2020 [252,253].
In summary, cells and EVs are categorized as “biological medicinal products” and if cells and EVs are delivered through a biomaterial, such a product will then be classified as a Class III medical device. With committed regulatory guidelines, the future development of the cell/EV-based medicinal products show promises to come closer to patients while maintaining quality, safety and efficacy.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23084074
This entry is offline, you can click here to edit this entry!
