Cancers affecting the gastrointestinal system are highly prevalent and their incidence is still increasing. Among them, gastric and pancreatic cancers have a dismal prognosis (survival of 5–20%) and are defined as difficult-to-treat cancers. This reflects the urge for novel therapeutic targets and aims for personalised therapies. As a prerequisite for identifying targets and test therapeutic interventions, the development of well-established, translational and reliable preclinical research models is instrumental. This review discusses the development, advantages and limitations of both patient-derived organoids (PDO) and patient-derived xenografts (PDX) for gastric and pancreatic ductal adenocarcinoma (PDAC). First and next generation multicellular PDO/PDX models are believed to faithfully generate a patient-specific avatar in a preclinical setting, opening novel therapeutic directions for these difficult-to-treat cancers.
1. Introduction
The survival of cancer patients has significantly improved during the last decade as a result of a better understanding of cancer biology and development of novel promising therapies, such as immunotherapy. Despite that, cancer incidence is still increasing, and cancer is a major cause of death worldwide. However, unfortunately, some cancer types still show high relapse and mortality rates. Gastric and pancreatic cancers, two types of gastrointestinal cancer, are known to have limited treatment options, progress quickly and result in high mortality [
1]. This emphasises the need for novel and effective therapeutic interventions. Previous cancer models have mainly relied on monolayer in vitro cell cultures and subcutaneous cellular grafting in immunodeficient mice. During the last decade, major advances have been made with the development of patient-derived organoid (PDO) cultures and engraftment of freshly isolated human tumour tissues in mice (patient-derived xenografts, PDXs). While differences exist regarding costs, scalability and the contribution of the tumour microenvironment (TME), these models benefit from a high degree of biological concordance and stability over time. Still, a lot of work needs to be done to completely recapitulate all elements of a human tumour in preclinical research.
2. Difficult-to-Treat Gastrointestinal Cancers
Gastric cancer and pancreatic cancer are some of the most lethal cancers known, with both occurring at a relatively high incidence. The dismal prognosis is partly due to late diagnosis and the limited treatment options currently available. In both of these cancers, neoplastic cells are surrounded by a significant amount of non-epithelial/stromal cells, such as cancer-associated fibroblasts (CAFs), infiltrating immune cells and endothelial cells forming the tumour blood vessels, all in a dense extracellular matrix. This TME favours tumour growth and progression and it functions as a physiological barrier, hampering oxygen supply and the delivery of therapeutics.
3. First Generation PDO/PDX Modelling
Historically, two-dimensionally (2D) grown cell lines derived from human tumour tissues have been used for cancer research for many years. Being relatively simple, stable, immortal, and inexpensive, cell-line based studies have shown significant value and have led to insights in oncologic signaling and the identification of novel therapeutic targets [
31]. Regardless, extrapolating preclinical findings to the clinic has become a major challenge in drug discovery because 2D models only partly represent the in vivo situation. Not all cancer cell subclones are reflected accurately in a (clonal) cell line and, importantly, there is a lack of the TME, resulting in no 3D cell–cell interactions and/or tumour–stroma interactions [
31,
32]. Culture conditions are also known to propagate phenotypic features that are not necessarily representative of the in vivo situation [
33]. Moving forward, inclusion of patient-derived 3D models has significantly improved the representation of the in vivo situation. Here, we review PDO and PDX to better model gastric and pancreatic cancer.
3.1. Patient-Derived Organoids (PDOs)
A major breakthrough in oncology research was the discovery of how to establish human 3D organoids from human tissues and cancers [
34]. Long-term murine and human organoid cultures have been generated from stem cells by stimulation of the Wnt pathway and inhibition of the Transforming Growth Factor (TGF)-β pathway [
35]. Cells obtained from surgical resections and biopsies of human tumours have been successfully used to establish PDOs with high representativeness of the original tumour on a morphological, genetic, and phenotypic level [
36,
37,
38]. Due to the method of culturing, PDOs have certain advantages over other cancer models (
Table 1). Compared to in vivo experiments, PDOs can be generated in small volumes and at low costs. It normally takes two weeks to one month from tissue digestion until a stable PDO culture is established [
38]. Culture conditions make PDOs fit for transcriptome and high-throughput drug screening, allowing in-depth studies in development and diseases, but also offering novel possibilities for more personalised treatment [
39,
40]. As an important drawback, the success rate of culturing PDO is highly dependent on the tumour origin, cellularity, tissue size, amount of stroma, and whether the patient received neoadjuvant therapy or not [
41]. This demands stan-darisation of protocols to create highly representative living biobanks for these and other cancer indications.
Table 1. Overview of the features of the various patient-derived models.
| Features |
PDO |
PDX |
Multicellular PDO |
Humanised PDX |
| Patient tumour recapitulation |
+ |
+ |
++(+) * |
++ |
| Presence of TME |
− |
+ |
−/+ |
++ |
| Model stability |
++ |
++ |
? |
? |
| Establishment time |
++ |
− |
++ |
− |
| Scalability |
++ |
+ |
? |
− |
| Costs |
low |
high |
low |
very high |
3.2. Patient-Derived Xenografts (PDXs)
One of the disadvantages of PDOs is that they do not fully represent the clinical situation, mainly due to the lack of the tumour stroma/TME and blood flow (
Table 1). To be able to evaluate this, PDX studies have been performed in which a human tumour is grown in an immunodeficient mouse. Primary (tumour) tissue, obtained via surgical resection or biopsy [
56,
57], is engrafted heterotopically (often subcutaneously) or orthotopically in the organ of tumour origin. Subcutaneous implantation is a relatively simple, less invasive method and growth can easily be monitored in vivo. However, heterotopic PDX tumours represent the former tumour environment less accurately and do not facilitate invasion and metastasis to a similar extent, compared to orthotopic transplantation [
58]. As a drawback, orthotopic PDX is often more difficult to monitor in vivo [
59]. Once PDX engraftment and growth is established, the tissue can be passed to new mice, expanding the amount of tumour tissue for subsequent analysis [
60]. Several researchers describe a phenomenon of serial passages having a shorter latency, which increases the tumour take rates [
56,
61]. This might be related to the formation of the required mouse vasculature in the PDX tissue after the first passage [
62], thereby accelerating subsequent tumour growth. Different PDX generations show a 95–100% concordance for histology and differentiation status. Research also shows high concordance of key driver mutations in PDXs and their corresponding human primary tumours [
63]. Retention of the original tumour characteristics makes PDX a more accurate model to predict therapeutic responses, compared to traditional cell-line based or non-human based animal models.
Apart from the advantages, there are some challenges associated with PDX models (
Table 1). Firstly, tumour growth after engraftment generally has a low efficiency. Factors that influence the success rate are tissue metabolism, ischemia time, sample size, surgical procedure, tumour type, tumour stage, metastatic properties, and mouse strain [
64,
65]. Another limitation is that, after engraftment of patient tissue into a mouse, the patient-derived stromal and immune cells do not proliferate [
66], resulting in their replacement with murine stromal cells. These cells are fully functional, but this hampers the use of PDX when it comes to evaluation of stroma-targeted therapies. Furthermore, the long time (from months to over one year) it takes to generate the primary PDX model limits the potential of the model in various research and clinical applications, such as personalised medicine.
In summary, PDX models allow increased understanding of tumour growth and dynamic tumour- (mouse) stroma interactions that regulate invasiveness and distant metastasis. Moreover, the high histological resemblance provides new oncological insights and opens interesting diagnostic, prognostic, and therapeutic applications towards more personalised medicine for difficult-to-treat cancers.
4. Next Generation PDO/PDX Modelling
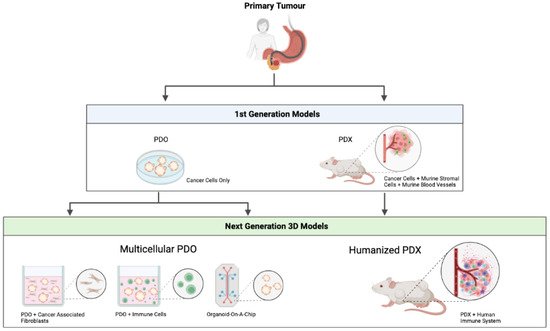
Irrespective of the promising PDO and PDX models that were discussed above, these models require further optimisation before broader clinical implementation becomes possible. Recent progress has been made to develop complex organotypic PDO/PDX-based models to closely mimic the complexity of a human tumour. An overview of the first and next generation patient-derived models can be seen in Figure 1.
Figure 1. Overview of first and next generation patient-derived models. PDO—patient-derived organoid. PDX—patient-derived xenograft. Figure created with
BioRender.com.
4.1. Multicellular PDO Models
Despite all of their advantages compared to conventional 2D cell culture, current PDO models do not fully recapitulate the complex cellular environment of a human cancer, since they lack the TME, a crucial component when investigating tumour development, progression and treatment options. Given the increasing efficacy of immunotherapy, the development of multicellular in vitro models involving infiltrating immune cells has gained interest. Adding immune cells to the PDO models was reported for the first time in the context of colorectal cancer and non-small-cell lung cancer [
83]. Hereby, peripheral T-cells were added to tumour PDO cultures, resulting in the enrichment of tumour reactive T-cells that were able to specifically kill the tumour PDO [
84]. As TME also contains other important elements, such as CAFs, blood vessels and neurons, in vitro modelling is currently moving forward to complex organotypic systems with close-to-human representativity.
4. Next Generation PDO/PDX Modelling
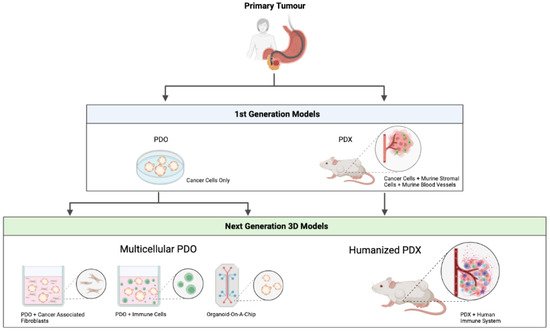
Irrespective of the promising PDO and PDX models that were discussed above, these models require further optimisation before broader clinical implementation becomes possible. Recent progress has been made to develop complex organotypic PDO/PDX-based models to closely mimic the complexity of a human tumour. An overview of the first and next generation patient-derived models can be seen in Figure 1.
Figure 1. Overview of first and next generation patient-derived models. PDO—patient-derived organoid. PDX—patient-derived xenograft. Figure created with
BioRender.com.
4.1. Multicellular PDO Models
Despite all of their advantages compared to conventional 2D cell culture, current PDO models do not fully recapitulate the complex cellular environment of a human cancer, since they lack the TME, a crucial component when investigating tumour development, progression and treatment options. Given the increasing efficacy of immunotherapy, the development of multicellular in vitro models involving infiltrating immune cells has gained interest. Adding immune cells to the PDO models was reported for the first time in the context of colorectal cancer and non-small-cell lung cancer [
83]. Hereby, peripheral T-cells were added to tumour PDO cultures, resulting in the enrichment of tumour reactive T-cells that were able to specifically kill the tumour PDO [
84]. As TME also contains other important elements, such as CAFs, blood vessels and neurons, in vitro modelling is currently moving forward to complex organotypic systems with close-to-human representativity.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23063147